The National Toxicology Program (NTP) Interagency Center for the Evaluation of Alternative Toxicological Methods(https://ntp.niehs.nih.gov/pubhealth/evalatm/) (NICEATM) joined with other government agencies and international partners to apply advanced nonanimal technologies to COVID-19 research. The Microphysiological Systems for COVID-19 Research (MPSCoRe) Working Group, which held its first meeting Jan. 29, is co-chaired by NICEATM Acting Director Nicole Kleinstreuer, Ph.D.
Microphysiological systems(https://ntp.niehs.nih.gov/whatwestudy/niceatm/test-method-evaluations/mps/) (MPS) — sometimes referred to as organs-on-chips — use human cells and engineered structures to create an environment that mimics, or models, the function of organs such as lungs (see sidebar).
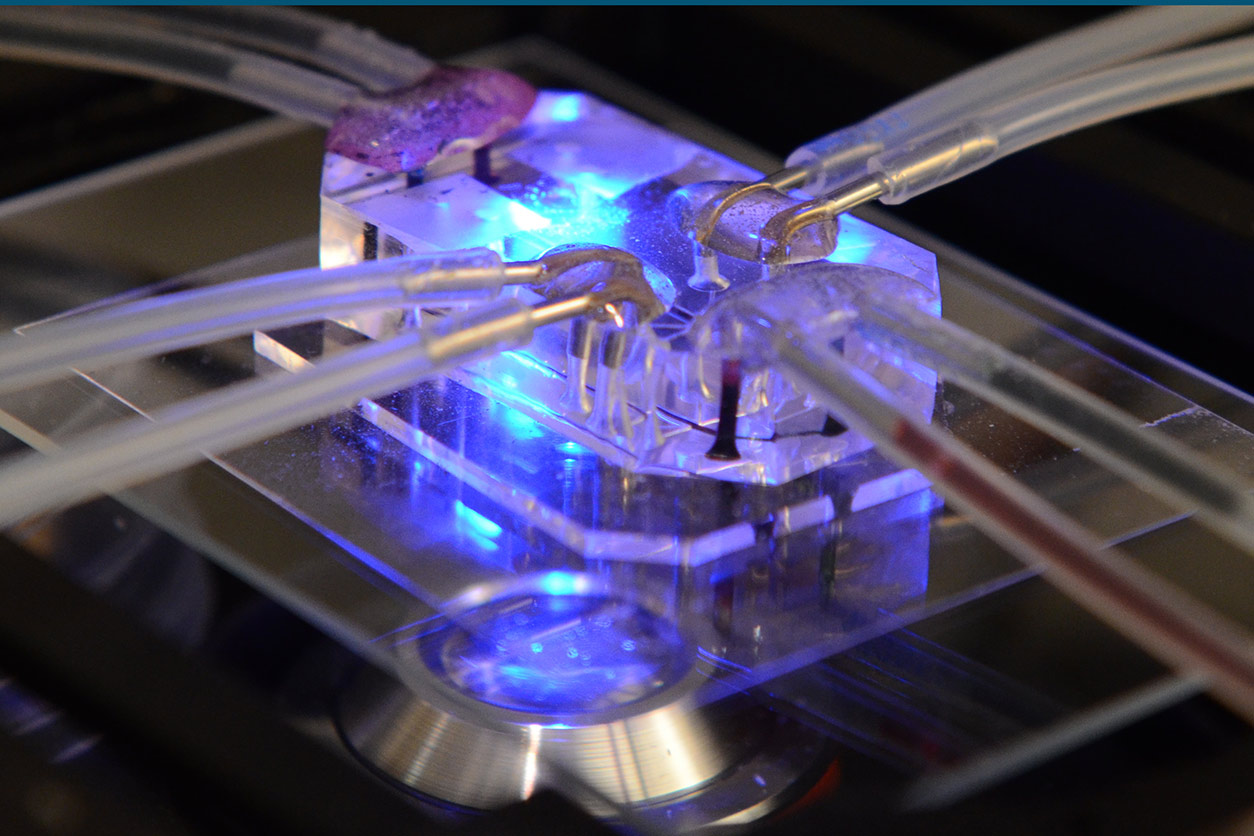 This 2011 photo show a MPS that models lung function for drug testing. (Photo courtesy of Wyss Institute at Harvard University, Creative Commons License CC BY-NC 2.0)
This 2011 photo show a MPS that models lung function for drug testing. (Photo courtesy of Wyss Institute at Harvard University, Creative Commons License CC BY-NC 2.0)The new working group will coordinate global use of MPS in research on the COVID-19 disease process and potential treatments. The group has more than 70 members representing government agencies, biotech and pharmaceutical companies, and academic institutions.
 “Using MPS will enable new medicines to be tested more rapidly and cheaply than traditional animal studies,” Kleinstreuer said. (Photo courtesy of Steve McCaw / NIEHS)
“Using MPS will enable new medicines to be tested more rapidly and cheaply than traditional animal studies,” Kleinstreuer said. (Photo courtesy of Steve McCaw / NIEHS)Human-based models needed
The limitations of animal models for studying COVID-19 were one area of discussion at the January meeting. Animals such as monkeys, mice, and hamsters tend to develop milder disease symptoms than humans, if they get sick at all, making it hard to test new medicines.
Kleinstreuer noted other compelling reasons for using human-based models in this research. “MPS will let us better understand how the SARS-CoV-2 virus interacts with human organs and elicits an immune response,” she said. “They can also be used to test drug efficacy in genetically diverse human populations.”
Coordinating across sectors
The urgent need for COVID-19 treatments has sparked rapid development of many different MPS models for this purpose, raising the possibility of fragmentation and resource duplication.
“This group will bring together model developers, drug and vaccine manufacturers, and regulators,” noted working group co-chair Anthony Holmes, Ph.D., from the U.K. National Centre for the Replacement, Refinement & Reduction of Animals in Research (NC3Rs). “This will enable representatives of different sectors to determine how MPS can best be used to bring new COVID-19 treatments forward using human-relevant systems that reduce our reliance on animal models.”
In addition to NICEATM and NC3Rs, the working group includes founding members from the National Center for Advancing Translational Sciences, the National Institute of Allergy and Infectious Diseases, and the U.S. Department of Defense.
 Holmes is the NC3Rs director of science and technology. (Photo courtesy of NC3Rs)
Holmes is the NC3Rs director of science and technology. (Photo courtesy of NC3Rs)First, compile data and research
High-quality data is needed to evaluate different MPS technologies for COVID-19 studies. A new data portal will be developed in the University of Pittsburgh Microphysiology Systems Database. The COVID-19 portal will receive financial support from NICEATM.
Attendees at the working group meeting were asked to add models and information to the University of Pittsburgh database. The group is also compiling relevant research. Information about MPSCoRe working group activities is available on the NTP website(https://ntp.niehs.nih.gov/go/mps).
(Catherine Sprankle is a communications specialist for ILS, the contractor supporting NICEATM.)