A new publication by scientists in the NIEHS Division of Translational Toxicology (DTT), colleagues from the German Federal Institute for Risk Assessment (BfR), and other U.S. government agencies describes the largest set of human data ever assembled for the purpose of evaluating nonanimal approaches for chemical safety testing.

The database has already been used to support international acceptance of a nonanimal strategy for identifying skin sensitizers, which are substances with the potential to cause an allergic reaction that results in an itchy rash, according to lead author Judy Strickland, Ph.D.
“We’re very excited to make it available to any researcher for developing and evaluating additional test methods,” she said.
International interest to address a pressing need
Regulatory agencies around the world require chemical products to be tested to determine whether they might be skin sensitizers. Because limitations or bans on using animals for new safety testing are becoming increasingly common, there is worldwide interest in seeking nonanimal approaches for identifying sensitizers.
DTT scientist Nicole Kleinstreuer, Ph.D., who is a coauthor on the paper, noted that computational and cell-based methods show promise for replacing animals for chemical safety testing.

“But high-quality data, preferably from humans, are needed to demonstrate that new methods can reliably identify whether a chemical can be a human sensitizer or cause other toxic effects,” she added. Kleinstreuer directs the National Toxicology Program Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM), which develops and evaluates alternatives to animal use for chemical safety testing.
NICEATM interacts frequently with international partners to advance evaluation and international adoption of new chemical safety testing approaches. For this project, NICEATM scientists collaborated with colleagues in the BfR, as well as in the U.S. Food and Drug Administration and the Consumer Product Safety Commission.
A curated, characterized database
The project started with human data that had been gathered by NICEATM over many years for evaluation of other testing approaches for skin sensitization. BfR played a critical role in collaborating with NICEATM to create the database, with the initial goal of supporting international acceptance of defined approaches developed under the umbrella of the Organization for Economic Co-operation and Development. Defined approaches use data from multiple sources to determine whether a chemical might be a skin sensitizer.
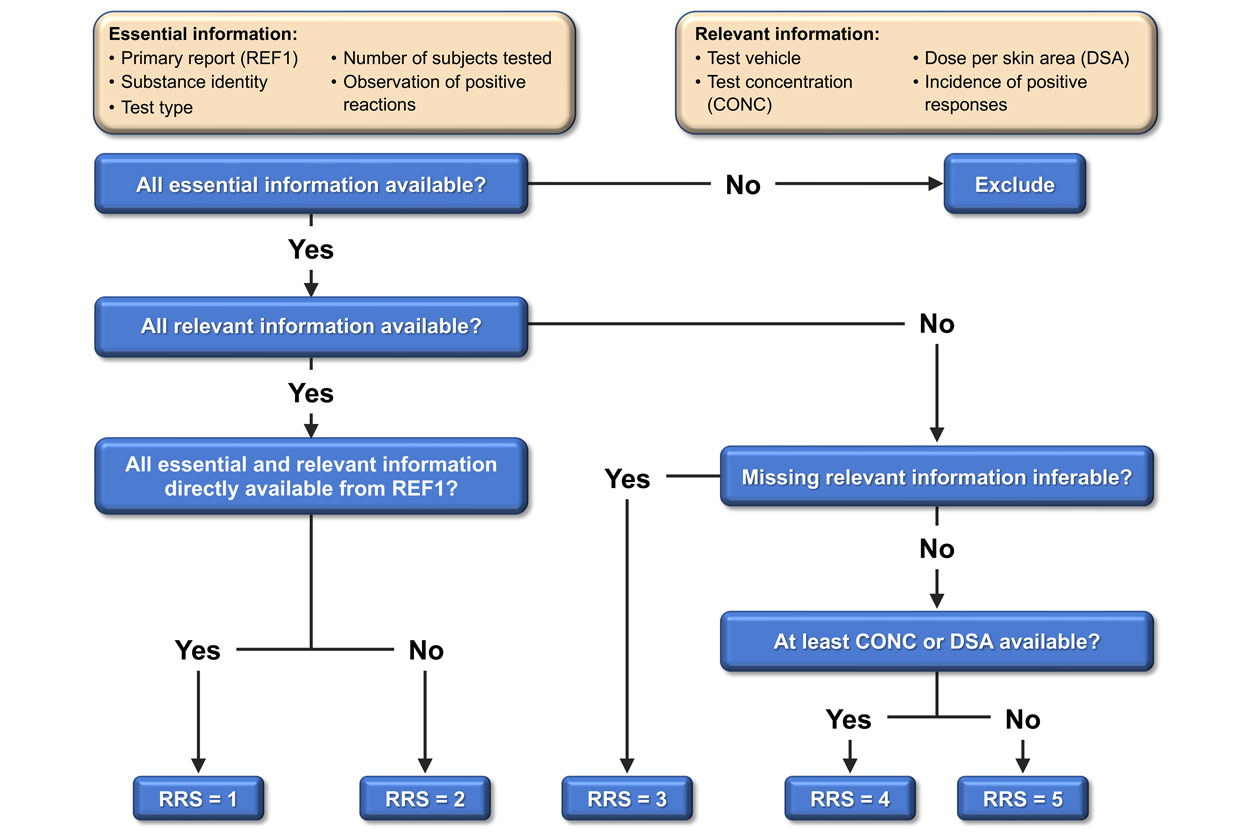
Ensuring that such data are of high quality takes a lot of work, explained BfR’s Matthias Herzler, Ph.D., senior author on the paper.
“We recorded relevant protocol elements and positive and negative outcomes and developed a scoring system to evaluate each test for reliability,” he noted. “We also traced each test result back to its original report to remove duplicates.”
The data described in the paper are available via the NICEATM Integrated Chemical Environment, an openly available source of curated chemical property and bioactivity data.
(Catherine Sprankle is a communications specialist for Inotiv, the contractor supporting NICEATM.)