 Lazar also directs the Institute for Diabetes, Obesity, and Metabolism at the University of Pennsylvania Perelman School of Medicine. (Photo courtesy of Mitchell Lazar)
Lazar also directs the Institute for Diabetes, Obesity, and Metabolism at the University of Pennsylvania Perelman School of Medicine. (Photo courtesy of Mitchell Lazar)The latest NIEHS Distinguished Lecture focused on how an organism’s circadian rhythms, or the physiological processes that regularly change based on a 24-hour period, influence metabolism.
Mitchell Lazar, M.D., Ph.D., the Willard and Rhoda Ware Professor in Diabetes and Metabolic Diseases at the University of Pennsylvania Perelman School of Medicine, presented 'Nuclear Receptors, Circadian Rhythms, and Metabolism' Oct. 13 using the Zoom platform.
Two nuclear receptors, Rev-erb and PPARgamma, are at the core of Lazar’s research. Nuclear receptors are proteins that bind to DNA and regulate cellular processes.
The first part of his talk focused on the liver, genes that control cellular clocks, and Rev-erb, a receptor he discovered.
'Throughout his career, Mitch Lazar’s research has focused on investigating mechanistic basic science questions and applying the findings to physiological questions,' said Kenneth Korach, Ph.D., head of the NIEHS Receptor Biology Group, who hosted the webinar.
'He has then applied these questions to biomedical conditions and diseases with a goal of developing diagnostic and therapeutic approaches.'
The liver’s clock
Circadian rhythms are generated by an organism’s internal clocks. The brain contains the central clock, which synchronizes the other clock genes that appear in almost every cell in the body. Lazar focuses on clock genes in the liver because diet-induced obesity changes how the proteins made from these genes work, which has a bearing on metabolism.
Hepatocytes make up approximately 80% of the cells in the liver and are responsible for the major functions of the organ, making bile, producing proteins, and detoxifying the body. The liver also contains stem cells, immune cells known as Kupffer cells, and endothelial cells.
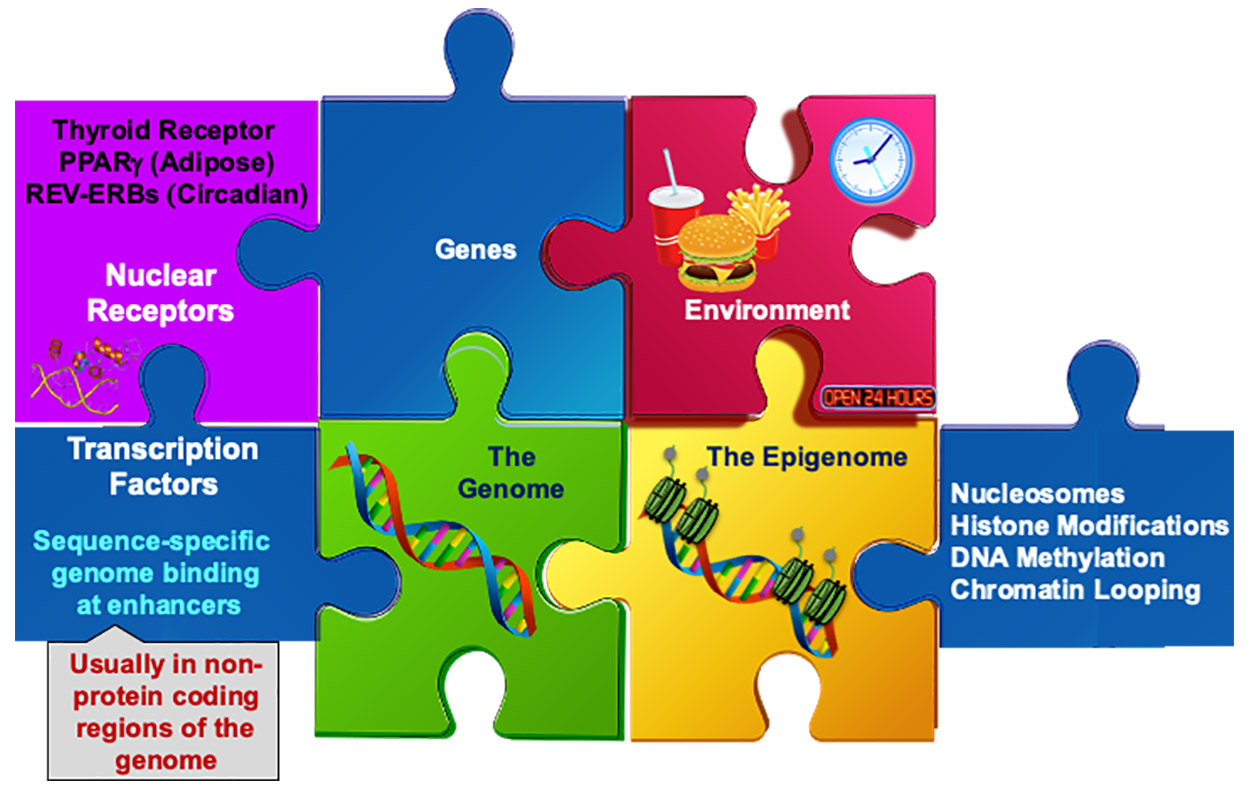 Lazar maintains that several puzzle pieces come together to influence circadian rhythms and metabolism. (Illustration courtesy of Mitchell Lazar)
Lazar maintains that several puzzle pieces come together to influence circadian rhythms and metabolism. (Illustration courtesy of Mitchell Lazar) Lazar knocked out Rev-erb in the hepatocytes of one group of mice and compared their gene expression or protein production to control mice hepatocytes that had Rev-erb. He was surprised to see that knockout mice exhibited gene expression changes in non-hepatocytes, such as Kupffer cells.
'The hepatocyte clock controls both the hepatocyte and non-hepatocyte circadian rhythms and metabolism,' Lazar said.
Path toward personalized medicine
He next discussed PPARgamma, the nuclear receptor required to make fat cells function properly. Lazar explained that mutations in PPARgamma can cause lipodystrophy, or the abnormal distribution of fat in the body, and insulin-resistant diabetes.
Several years ago, pharmaceutical companies started looking for compounds that would bind to PPARgamma in hope of finding a diabetes drug. One of the molecules that held the most promise was rosiglitazone. Although it dramatically improved insulin resistance, it did not reduce the risk of heart attack and stroke, in part due to its undesired effect of increasing cholesterol levels.
Lazar and his team determined that a specific small difference in the genome, called a single nucleotide polymorphism, was a genetic determinant of whether a given patient would experience the unwanted side effect of rosiglitazone. Subsequent studies proved the hypothesis correct (see sidebar).
'My suggestion is this example is generalizable to other nuclear receptors, like corticoids and estrogens and others, but it could be for all drugs that work as transcription factors,' Lazar said. 'It’s a path toward personalizing medicine, in this case, based on a fundamental knowledge of genomics and biological mechanisms.'









