NIEHS, Duke University, and the University of North Carolina at Chapel Hill (UNC) joined forces in 2017 with the launch of the Molecular Microscopy Consortium (MMC). The goal: to support scientific research by providing training on, and access to, cryogenic electron microscopy, or cryo-EM. During the first Triangle Area Cryo-EM Symposium, held Dec. 16-17 in Research Triangle Park, group members celebrated their recent collaborative successes.
 Zeldin said the MMC has far exceeded expectations and is well ahead of schedule in terms of equipment and publications. (Photo courtesy of Steve McCaw)
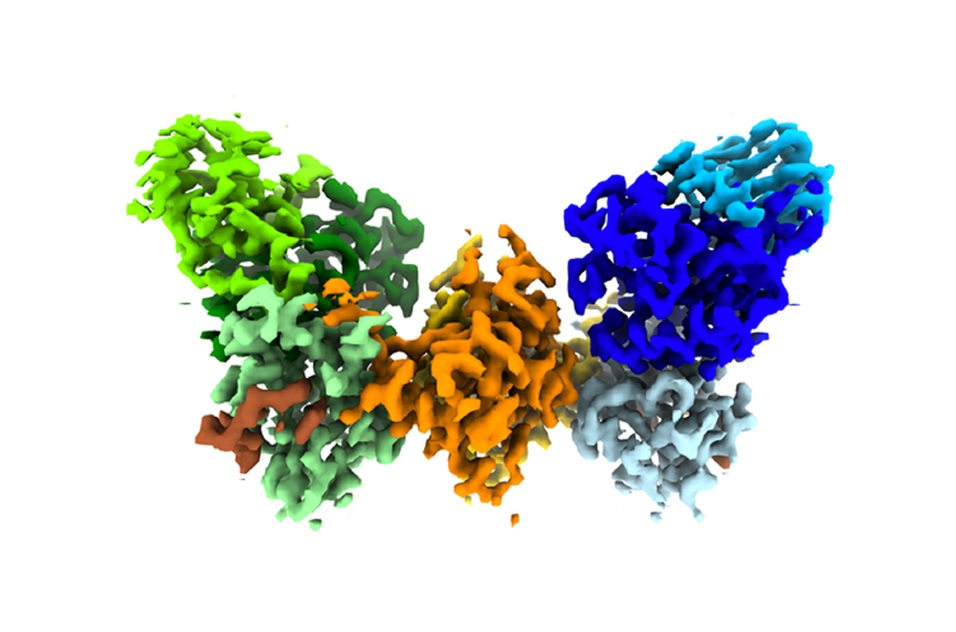
Zeldin said the MMC has far exceeded expectations and is well ahead of schedule in terms of equipment and publications. (Photo courtesy of Steve McCaw)Cryo-EM shows the shape of biomolecular complexes, which are made of multiple molecules of protein, DNA, RNA, lipids, or carbohydrates, and combinations thereof. The method has revolutionized structural biology, which traditionally relied on X-ray crystallography, and increasingly is used in environmental health research.
At the meeting, 27 speakers — most of whom are part of the consortium — presented their cryo-EM work to more than 100 attendees.
“Earlier this year, the first three papers resulting from MMC efforts were published in Science, Cell Reports, and Nature Communications, one from each of the member institutions,” said NIEHS Scientific Director Darryl Zeldin, M.D., in his opening remarks. “Those papers were followed by another half dozen manuscripts over the next few months.”
Such achievements are due in part to the deployment of cryo-EM instruments at NIEHS, Duke, and UNC, and the training of scientists on how to incorporate the technology into their research. The group is gaining attention elsewhere — symposium participants included researchers from North Carolina State University and East Carolina University — and opening the door to more collaboration.
“We have accomplished together what could not have been accomplished by one institution working alone,” said Zeldin.
Technology on the rise
Cryo-EM was named the 2015 Method of the Year by Nature Methods. And in 2017, three pioneers in the field won the 2017 Nobel Prize in Chemistry.
 Borgnia’s numerous contributions to cryo-EM research and training were cited in many of the conference presentations. (Photo courtesy of Steve McCaw)
Borgnia’s numerous contributions to cryo-EM research and training were cited in many of the conference presentations. (Photo courtesy of Steve McCaw)Earlier that year, NIEHS recruited Mario Borgnia, Ph.D., from the National Cancer Institute to organize the MMC. He opened the NIEHS cryo-EM facility, which was the first in the Carolinas (see video, first sidebar).
“[The imaging] allows us to determine the structure of things that we couldn’t get by X-ray crystallography,” said Borgnia.
It also helps researchers learn how molecular structures function and influence biological systems. That knowledge can lead to innovations in drugs and vaccines, and expand basic mechanistic understanding of how diseases work (see second sidebar).
Pharmaceutical implications
Plenary speaker Robert Lefkowitz, M.D., of Duke, who shared the 2012 Nobel Prize for Chemistry, discussed how he uses cryo-EM to build on his previous research.
In the 1980s, he identified G-protein-coupled receptors (GPCRs), the largest family of targets for drugs. More than 700 pharmaceuticals, or about one-third of all Food and Drug Administration-approved drugs, are known to target GPCRs.
 Lefkowitz is James B. Duke Distinguished Professor of Medicine at Duke. (Photo courtesy of Steve McCaw)
Lefkowitz is James B. Duke Distinguished Professor of Medicine at Duke. (Photo courtesy of Steve McCaw)“So, this basic science project has had echoes throughout the practice of medicine,” said Lefkowitz.
Today, his team uses cryo-EM to study previously unknown structures called megaplexes, in which a GPCR is bound with other cellular and molecular components.
 Acharya is Director of the Department of Structural Biology at the Duke Human Vaccine Institute. (Photo courtesy of Steve McCaw)
Acharya is Director of the Department of Structural Biology at the Duke Human Vaccine Institute. (Photo courtesy of Steve McCaw)Lefkowitz said that as he continues his work with more complex GPCR-related molecules, he will be interested in building larger structures for cryo-EM analysis.
NIEHS and consortium contributors
NIEHS presenters described their diverse cryo-EM projects.
The symposium was organized by MMC members Borgnia and Stanley from NIEHS; Priyamvada Acharya, Ph.D., and Alberto Bartesaghi, Ph.D., from Duke; and Saskia Neher, Ph.D., and Matthew Redinbo, Ph.D., from UNC.
(Ernie Hood is a contract writer for the NIEHS Office of Communications and Public Liaison.