Suk directs SRP, which fosters interdisciplinary research approaches to address the problems associated with potentially hazardous environmental exposures. (Photo courtesy of Steve McCaw)
Suk directs SRP, which fosters interdisciplinary research approaches to address the problems associated with potentially hazardous environmental exposures. (Photo courtesy of Steve McCaw)The challenge of studying the effects of exposures when those effects do not appear for years or even decades was the topic of a Superfund Research Program (SRP) webinar series that concluded June 16. A long lag time, or latency, between exposure and the onset of diseases such as cancer can make it difficult for scientists to discover the links between exposures, molecular changes in the body, and later-life health outcomes.
“By bringing together experts working on issues in this field, we hope that the series sparks new ideas and helps build on concepts related to exposures and latent disease risk,” said SRP Director Bill Suk, Ph.D.
“Latency complicates traditional views of toxicology,” said Brian Berridge, D.V.M., Ph.D., scientific director of the NIEHS Division of the National Toxicology Program (DNTP). He spoke May 11 in the first session of the series. “The more I dug into the concept of exposure and latent disease risk, the more complex the subject became, particularly in the context of how to model and ultimately predict latent hazards,” he noted.
Berridge explained the rapid increase in chemicals and agents in the environment calls for a move from predominately observational toxicology to a more predictive approach, focusing on molecular mechanisms to identify hazards. Making such predictions is more challenging when health outcomes appear only decades later.
Predicting hazards and disease pathways
The need to identify chemical characteristics that predict potential biological effects emerged as a key theme during the series. For example, identifying key characteristics of carcinogens and endocrine disruptors provides a basis for organizing data on mechanisms of disease. These characteristics may allow chemical exposures to be linked to disease earlier in its development.
 Fry discussed her studies of prenatal exposure to arsenic at the 2018 NIEHS Global Environmental Health. (Photo courtesy of Steve McCaw)
Fry discussed her studies of prenatal exposure to arsenic at the 2018 NIEHS Global Environmental Health. (Photo courtesy of Steve McCaw)Using arsenic as a case study, researchers described how early life exposure can predict later health effects, including cancer and immune system changes. Increasing evidence suggests that latent diseases may be tied to changes to the epigenome, which are heritable changes in gene expression that occur with no alteration in DNA sequence.
“We have identified critical genes linked to both infant fetal growth and later life health outcomes,” said University of North Carolina at Chapel Hill (UNC) SRP Center Director Rebecca Fry, Ph.D. According to Fry, who spoke during the series kick-off session, understanding epigenetic regulation of these genes could help explain the mechanisms by which arsenic leads to both early and later life health outcomes. Her research team is developing strategies to integrate epigenetic data into the risk assessment process.
Rethinking the roles of time, aging
“If we are to overcome challenges in studying latency between risk factors and disease, we need to rethink the role of time in environmental health studies,” said Manish Arora, Ph.D., from the Icahn School of Medicine at Mount Sinai. He spoke during the June 16 webinar.
According to Arora, human metabolism is cyclical, so sampling study participants at discrete time points may miss dynamic responses to changes in the environment. He measures biomarkers in human baby and permanent teeth to reconstruct the timing of exposures to chemicals and essential nutrients. His team aims to develop predictive models that may prevent diseases decades before clinical signs become apparent.
Ron Kohanski, Ph.D., deputy director of the Division of Aging Biology at the National Institute on Aging (NIA), brought in the concept of aging as a risk factor of disease in his May 28 talk. “Clinically useful information about the trajectories of human aging can be obtained in two to three years of doctor’s visits,” he said.
In particular, molecular hallmarks of aging, such as changes to the immune system, can be used to understand interactions between the environment and aging, potentially providing opportunities to intervene and improve health.
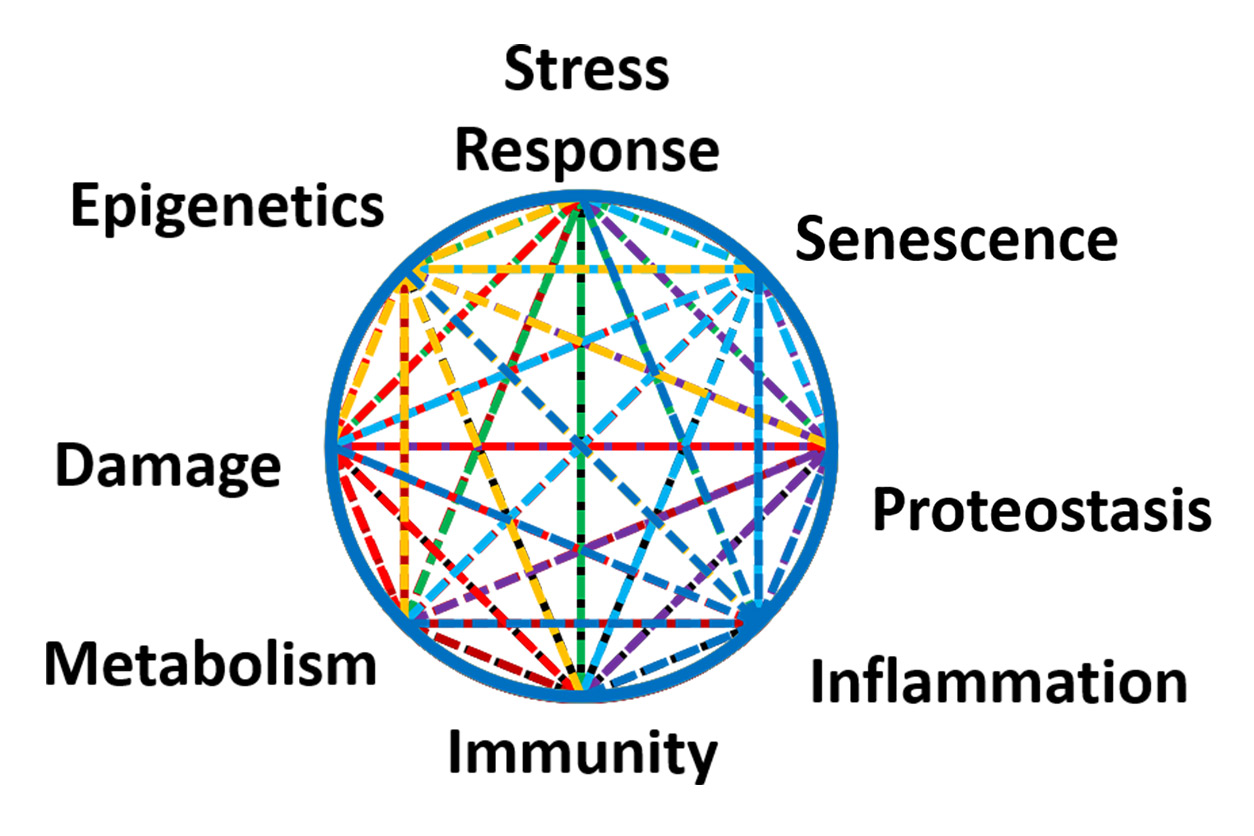 Kohanski described molecular hallmarks of aging, shown right, which are altered as people get older, leading to changes in different organ systems and a greater burden of chronic disease. (Photo courtesy of Ron Kohanski)
Kohanski described molecular hallmarks of aging, shown right, which are altered as people get older, leading to changes in different organ systems and a greater burden of chronic disease. (Photo courtesy of Ron Kohanski)Citations:
Smith MT, Guyton KZ, Kleinstreuer N, Borrel A, Cardenas A, Chiu WA, Felsher DW, Gibbons CF, Goodson WH, Houck KA, Kane A, La Merrill MA, Lebrec H, Lowe L, McHale CM, Minocherhomji S, Rieswijk L, Sandy MS, Sone H, Wang A, Zhang L, Zeise L, Fielden M. 2020. The key characteristics of carcinogens: relationship to the hallmarks of cancer, relevant biomarkers, and assays to measure them. Cancer Epidemiol Biomarkers Prev; doi:10.1158/1055-9965.EPI-19-1346 [Online 9 Mar 2020].
La Merrill MA, Vandenberg LN, Smith MT, Goodson W, Browne P, Patisaul HB, Guyton KZ, Kortenkamp A, Cogliano VJ, Woodruff TJ, Rieswijk L, Sone H, Korach KS, Gore AC, Zeise L, Zoeller RT. 2020. Consensus on the key characteristics of endocrine-disrupting chemicals as a basis for hazard identification. Nat Rev Endocrinol 16(1):45–57.
Rager JE, Auerbach SS, Chappell GA, Martin E, Thompson CM, Fry RC. 2017. Benchmark dose modeling estimates of the concentrations of inorganic arsenic that induce changes to the neonatal transcriptome, proteome, and epigenome in a pregnancy cohort. Chem Res Toxicol 30(10):1911–1920.
(Sara Amolegbe is a research and communication specialist for MDB Inc., a contractor for the NIEHS Superfund Research Program.)