Bebenek said polymerase mu is remarkable because the enzyme seems to have evolved to deal with unstable targets, such as double-strand DNA breaks. (Photo courtesy of Steve McCaw)
Bebenek said polymerase mu is remarkable because the enzyme seems to have evolved to deal with unstable targets, such as double-strand DNA breaks. (Photo courtesy of Steve McCaw)Our genomes are constantly bombarded by damage from natural and manmade chemicals, the sun’s ultraviolet rays, and other agents. If the cell’s DNA repair machinery does not fix this damage, our genomes can become dangerously unstable, which may lead to cancer and other diseases.
NIEHS researchers have taken the first snapshot of an important DNA repair protein — called polymerase mu — as it bridges a double-strand break in DNA. The findings, which were published Sept. 22 in Nature Communications, give insight into the mechanisms underlying DNA repair and may aid in the understanding of cancer and cancer therapeutics.
“Cancer cells depend heavily on this type of repair because they are rapidly dividing and especially prone to DNA damage,” said senior author Kasia Bebenek, Ph.D., a staff scientist in the institute’s DNA Replication Fidelity Group. “To understand how cancer originates and how to target it better, you need to know exactly how these individual DNA repair proteins work.”
Caught in the act
The most toxic form of DNA damage is the double-strand break, which is a cut that severs both strands of the double helix. Polymerase mu is one of a few enzymes that can help to repair these breaks, and it is capable of handling double-strand breaks that have jagged, unpaired ends.
A team led by Bebenek and Lars Pedersen, Ph.D., head of the NIEHS Structure Function Group, sought to take a picture of polymerase mu as it interacted with a double-strand break.
 Pedersen is an expert in x-ray crystallography, a technique that enables scientists to produce atomic-level, three-dimensional structures of molecules. (Photo courtesy of Steve McCaw)
Pedersen is an expert in x-ray crystallography, a technique that enables scientists to produce atomic-level, three-dimensional structures of molecules. (Photo courtesy of Steve McCaw)“It sounds simple, but it is actually quite difficult,” said Bebenek.
It can take thousands of tries to coax a protein out of solution and into an ordered crystal lattice that can be examined by X-rays.
Team member Andrea Kaminski, a biologist in Pedersen’s lab, has spent years studying the biochemistry of these enzymes and has developed the ability to crystallize these proteins both before and after the reaction occurs. These snapshots allowed the researchers to gain critical insight into the chemistry and how the enzyme makes repair of double-strand breaks possible.
Bridging the severed strands
The snapshots were striking. Polymerase mu formed a rigid structure that bridged the two severed strands of DNA.
Pedersen said the remarkable rigidity of the structure might allow polymerase mu to deal with the most unstable types of DNA breaks.
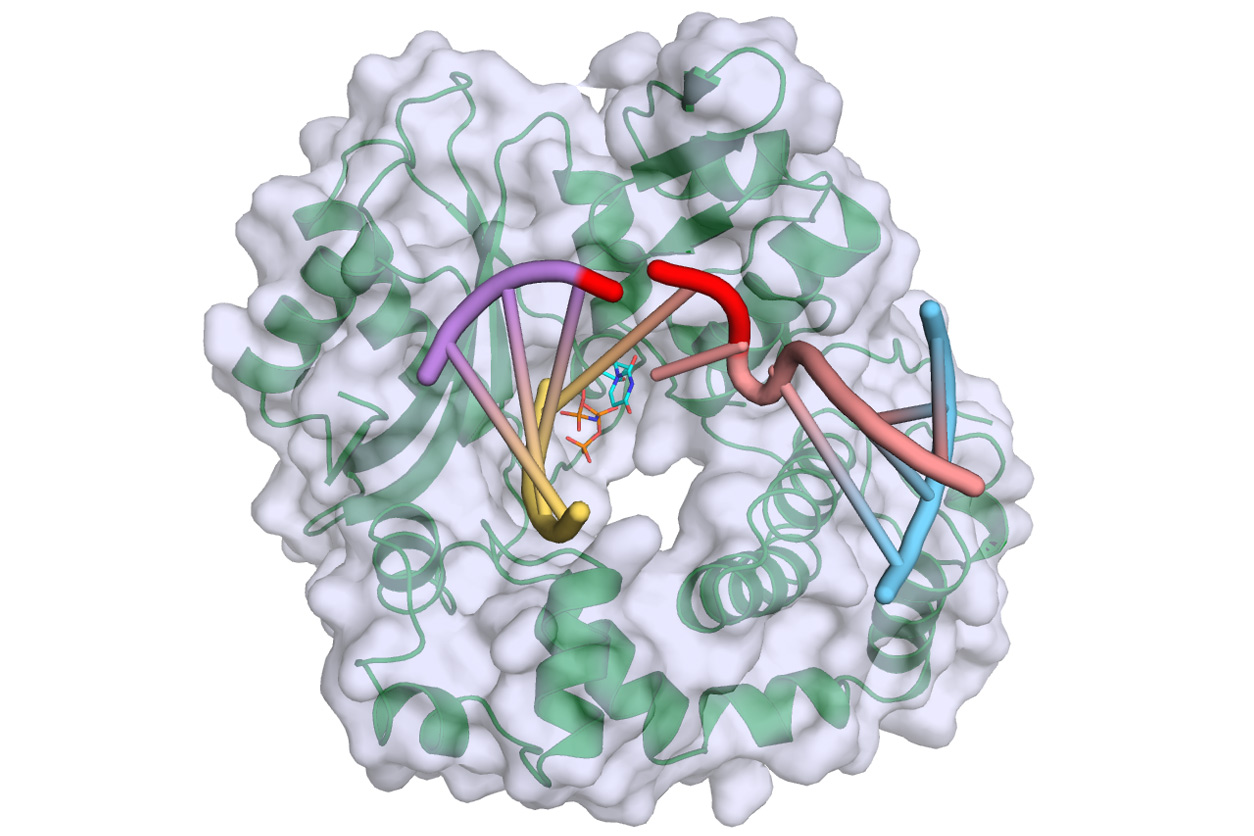 Polymerase mu — green, with gray surface — binds and bridges a DNA double-strand break, filling gaps at the break site, which is highlighted in red, with incoming complementary nucleotides, colored in cyan. Yellow and purple strands represent the upstream DNA duplex, and pink and blue strands represent the downstream DNA duplex. (Photo courtesy of NIEHS)
Polymerase mu — green, with gray surface — binds and bridges a DNA double-strand break, filling gaps at the break site, which is highlighted in red, with incoming complementary nucleotides, colored in cyan. Yellow and purple strands represent the upstream DNA duplex, and pink and blue strands represent the downstream DNA duplex. (Photo courtesy of NIEHS)“A running theme in our studies of polymerase mu is how little change it requires to handle a variety of different types of DNA damage,” he said.
However, polymerase mu does not act alone to repair breaks in DNA. Going forward, the researchers plan to understand how all the enzymes involved in this process work together to fill and seal the broken DNA strand to complete the repair.
Citation: Kaminski AM, Pryor JM, Ramsden DA, Kunkel TA, Pedersen LC, Bebenek K. 2020. Structural snapshots of human DNA polymerase mu engaged on a DNA double-strand break. Nat Commun 11(1):4784.
(Marla Broadfoot, Ph.D., is a contract writer for the NIEHS Office of Communications and Public Liaison.)