At the Sept. 2-3 meeting of the Scientific Advisory Committee on Alternative Toxicological Methods(https://ntp.niehs.nih.gov/go/sacatm) (SACATM), committee members praised advances in use of computational methods to reduce animal use for chemical safety testing. They suggested resources and approaches that could be developed to further advance these tools, and identified testing areas where they could be applied.
The committee encouraged member agencies of the Interagency Coordinating Committee on the Validation of Alternative Methods(https://ntp.niehs.nih.gov/go/iccvam) (ICCVAM) to increase efforts to advance nonanimal alternatives for identifying substances that could cause cancer or other chronic toxicity outcomes. They also encouraged agencies to promote strategies for reducing animal use for vaccine testing and antibody production (see sidebar).
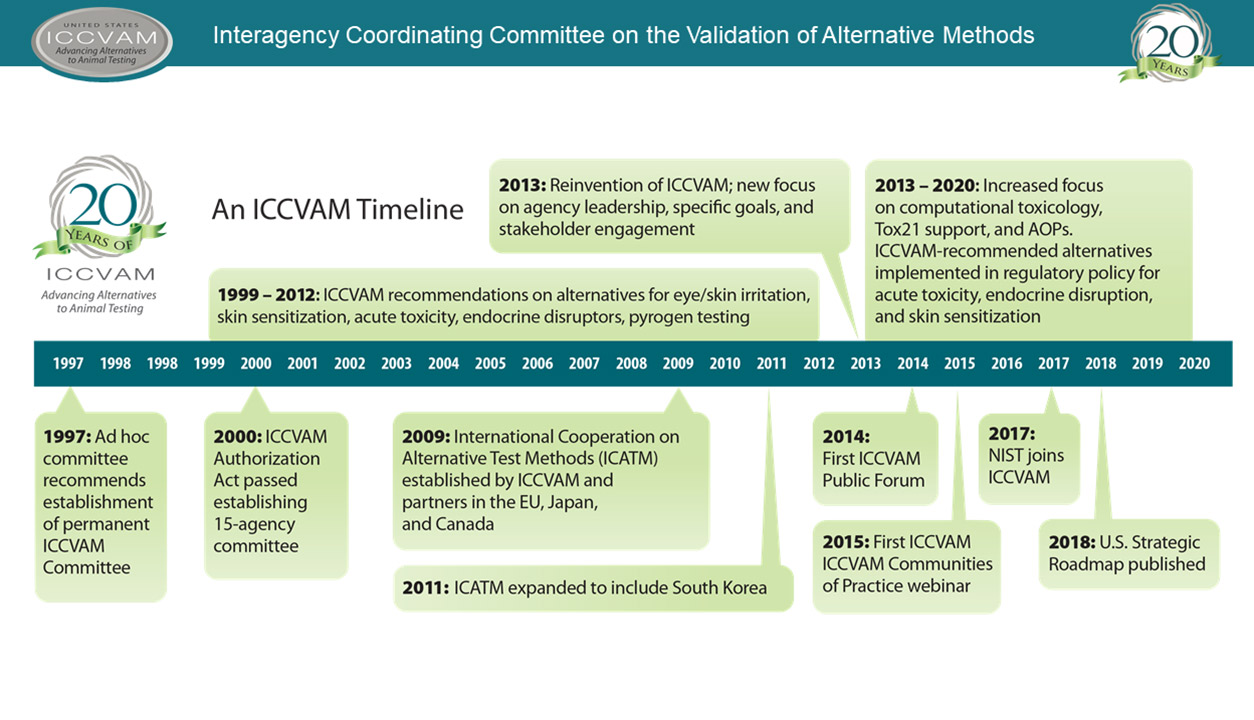
 Sixteen federal agencies make up ICCVAM. (Image courtesy of NIEHS)
Sixteen federal agencies make up ICCVAM. (Image courtesy of NIEHS)SACATM includes experts from academia, industry, and animal welfare organizations. The committee meets annually to advise the NIEHS director, ICCVAM, and the National Toxicology Program (NTP) Interagency Center for the Evaluation of Alternative Toxicological Methods(https://ntp.niehs.nih.gov/go/niceatm) (NICEATM), which administers ICCVAM.
Put good data to good use
An entire day of the meeting was devoted to considering the data needed to evaluate new methods and tools to put data to use.
 Kleinstreuer leads computational toxicology work and holds a secondary appointment in the NIEHS Biostatistics and Computational Biology Branch.
Kleinstreuer leads computational toxicology work and holds a secondary appointment in the NIEHS Biostatistics and Computational Biology Branch.Acting NICEATM Director Nicole Kleinstreuer, Ph.D., explained how curated data from animal and human studies are needed to develop new methods. “We rely on the curation of reference data,” she noted. She further explained that a large part of NICEATM activities involves assessing variability in data to set expectations for performance of alternative approaches.
Kleinstreuer shared examples of how repeated animal, or in vivo, tests for effects like eye or skin irritation often yield different results. In the presentation that followed, Katie Paul-Friedman, Ph.D., from the U.S. Environmental Protection Agency, showed that similar variation is observed in long-term animal studies.
The committee appreciated the importance of this work. “Something like this [repetition] should probably be required for all the in vivo data sets that are being used,” noted Tamara Tal, Ph.D., an ad hoc member from the German Helmholtz Centre for Environmental Research. She suggested that a symposium or white paper might increase awareness of this issue.
Later presentations provided overviews of computational tools for exploring chemical properties and generating toxicity predictions. SACATM members praised updates to the NICEATM Integrated Chemical Environment(https://ice.ntp.niehs.nih.gov/) web resource.
“I'm very impressed by the improvements and changes that have been made over the last year, and the level of curation and the thoughtfulness that has been given to the anticipated needs of the users,” commented SACATM member ClarLynda Williams-Devane, Ph.D., from the North Carolina Department of Health and Human Services.
Measuring success
Recently, ICCVAM has been considering how to measure and communicate success in reducing animal use for testing. Last year, members established its Metrics Workgroup to address that issue. Suzanne Fitzpatrick, Ph.D., the U.S. Food and Drug Administration’s lead representative on ICCVAM, shared updates on the workgroup, noting that its significance is reflected by having 19 members. They represent eight of the committee's 16 federal partners. “Agencies want to get their stories out and let stakeholders know what they’re doing.”
The workgroup is preparing a report on the agencies' various approaches to measuring use of alternative methods and reductions in animal testing.
SACATM member Sean Gehen, Ph.D., from Corteva Agriscience, joined with others concurring with the workgroup's importance. “It’s great to see the emphasis on metrics and taking into account actual animal use, and of course tracking that progress over time,” he said. “Hopefully, we can collectively identify what is working well in practice, and maybe things that aren't working well and continue to build upon that.”
Slides, minutes, and other materials from the SACATM meeting are available on the NTP website(https://ntp.niehs.nih.gov/events/past/).
(Catherine Sprankle is a communications specialist for ILS, the contractor supporting NICEATM.)