Research by Beate Ritz, M.D., Ph.D., from the University of California at Los Angeles, untangles complex interactions between genetic variation, pesticide exposure, and Parkinson’s disease. Such gene-environment interaction is termed G x E.
In Nov. 13 talk for the NIEHS Keystone Science Lecture Seminar Series, the longtime grantee shared findings from the Parkinson’s, Environment, and Genes (PEG) study. The seminar was hosted by Kimberly Gray, Ph.D., from the Population Health Branch. Reviewing Ritz’s many roles and honors, Gray said, “She has been an important member of the NIH [National Institutes of Health] extended family.”
 Ritz said what she loves about science is asking questions — and telling stories about what she finds. “You don’t always get answers, but you have to ask,” she said. (Photo courtesy of Steve McCaw)
Ritz said what she loves about science is asking questions — and telling stories about what she finds. “You don’t always get answers, but you have to ask,” she said. (Photo courtesy of Steve McCaw)Pesticides and the brain
In southern California in the 1980s, a so-called natural experiment shed light on pesticides’ neurological effects, when a heroin contaminant called MPTP caused Parkinson’s-like symptoms in users. Researchers discovered that MPTP killed dopamine neurons — the same neurons damaged in Parkinson’s disease.
“Parkinson’s disease is the second most common neurodegenerative disease, and there are no medications to slow its progression,” Ritz said. “We need to prevent it.” She showed that two pesticides have been linked to Parkinson’s — paraquat and a plant-derived pesticide called rotenone. Paraquat has a chemical structure similar to MPTP.
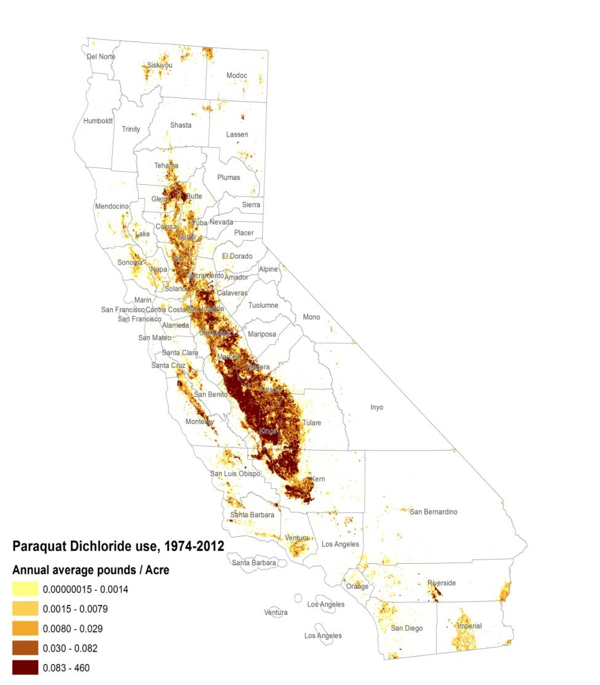 One of the maps Ritz shared shows typical distribution of pesticide use in California’s central valley, this one of paraquat. (Photo courtesy of Beate Ritz)
One of the maps Ritz shared shows typical distribution of pesticide use in California’s central valley, this one of paraquat. (Photo courtesy of Beate Ritz) “California is an AG [agriculture] state,” she said. “We are using 25 percent of all pesticides applied in the U.S.” Maps based on California’s Pesticide Use Reporting database show how use is concentrated in the state’s central valley. Residents live amid the rich farmland and the battery of chemicals used to manage it.
So that is where, in 2000, Ritz launched PEG, enrolling participants in Fresno, Tulare, and Kern counties.
E — environment
Collecting specific exposure data means looking into various scenarios.
- Low, long-term exposure, such as from well water.
- High-dose occupational exposure, including farmers and commercial pest control.
- Exposures from what is taken home on clothing, shoes, hair.
- Indoor and gardening pesticide use at home, which is the primary nonoccupational exposure.
- Residential proximity to agriculture and other pesticide applications.
Researchers gathered biosamples across seasons and years, but faced a complex task. “In California, there are 640 active ingredients tracked,” Ritz said. “We don’t even know about inactive ones, which may yet [be found to] cause DNA damage, immunotoxicity, [and] neurotoxicity or endocrine disruption.”
 Gray oversees grants programs in children’s health and environmental pediatric epidemiology. (Photo courtesy of Steve McCaw)
Gray oversees grants programs in children’s health and environmental pediatric epidemiology. (Photo courtesy of Steve McCaw)E x E
PEG researchers study effects of multiple chemicals — a challenge that is one focus area of the NIEHS Strategic Plan. For example, Ritz and colleagues knew that in the presence of the fungicide maneb, paraquat became more effective at killing dopamine neurons in mice. Thus, hers was the first study to show that a combination of maneb and paraquat exposures in this human population increased Parkinson’s risk.
“So the E of G x E also houses an E x E component,” she said. Added to combinations of chemicals and locations were combined exposures from different sources such as air, soil, and well water. “For well-water contamination, we’re seeing that if more than 10 water soluble pesticides were applied near wells, the risk for Parkinson’s was more than 70 percent increased [over baseline risk],” she said.
G is for genes
Turning to the G of the equation, Ritz explained that genes related to Parkinson’s disease risk from exposures code for metabolizers, transporters, DNA repair processes, and immune function.
For example, a gene called PON1 influences the breakdown of organophosphate pesticides (OPs). Some people have variations, or alleles, of that gene that result in slower OP metabolism. “Without exposure, you can be a poor metabolizer and it doesn’t matter,” Ritz said.
Individuals with the slow metabolizing allele who were also exposed to diazinon, chlorpyrifos, or parathion showed a 2.5- to 3-fold increase in Parkinson’s disease risk. They also experienced faster progression if they got the disease.
Ritz cheered the development of epigenetics — the study of changes to DNA, such as the addition of molecules called methyl groups, that alter its function without changing the underlying sequence of nucleotides. Many of these changes are heritable.
Now she and her colleagues are analyzing stored blood and saliva samples. They reported that individuals with Parkinson’s disease and individuals with OP exposures showed different methylation levels at certain genetic loci.
Ritz hopes that her studies will soon also be able to point to a biomarker for exposure to pyrethroids and nicotinoids, which are replacing OPs in agricultural use.
Many of the slides Ritz showed appear on her website.
 Folks from across the institute packed the room to hear about Ritz’s G x E work on Parkinson’s disease and pesticides. (Photo courtesy of Steve McCaw)
Folks from across the institute packed the room to hear about Ritz’s G x E work on Parkinson’s disease and pesticides. (Photo courtesy of Steve McCaw)Citations:
Chuang YH, Paul K, Bronstein J, Y. Bordelon, Horvath S, Ritz B. 2017. Parkinson's disease is associated with DNA methylation levels in human blood and saliva. Genome Med 9(1):76.
Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. 2009. Parkinson's disease and residential exposure to maneb and paraquat from agricultural applications in the central valley of California. Am J Epidemiol 169(8):919–26.
Gatto NM, Cockburn M, Bronstein J, Manthripragada AD, Ritz B. 2009. Well-water consumption and Parkinson's disease in rural California. Environ Health Perspect 117(12):1912–1918.
Lee PC, Rhodes SL, Sinsheimer JS, Bronstein J, Ritz B. 2013. Functional paraoxonase 1 variants modify the risk of Parkinson's disease due to organophosphate exposure. Environ Int 56:42–47..
Narayan S, Liew Z, Paul K, Lee PC, Sinsheimer JS, Bronstein JM, Ritz B. 2013. Household organophosphorus pesticide use and Parkinson’s disease. Int J Epidemiol 42(5):1476–1485.
Paul KC, Chuang YH, Cockburn M, Bronstein JM, Horvath S, Ritz B. 2018. Organophosphate pesticide exposure and differential genome-wide DNA methylation. Sci Total Environ 645:1135–1143.
Paul KC, Sinsheimer JS, Cockburn M, Bronstein JM, Bordelon Y, Ritz BR. 2017. Organophosphate pesticides and PON1 L55M in Parkinson's disease progression. Environ Int 107:75–81.











