This month, I am excited to share with you excerpts from my recent conversation with Cheryl Walker, Ph.D., a long-time NIEHS grant recipient who directs the Center for Precision Environmental Health at Baylor College of Medicine.
 In future months, look for more interviews like this one. I want to increase opportunities for collaboration, which starts with conversation and the exchange of ideas. (Image courtesy of NIEHS)
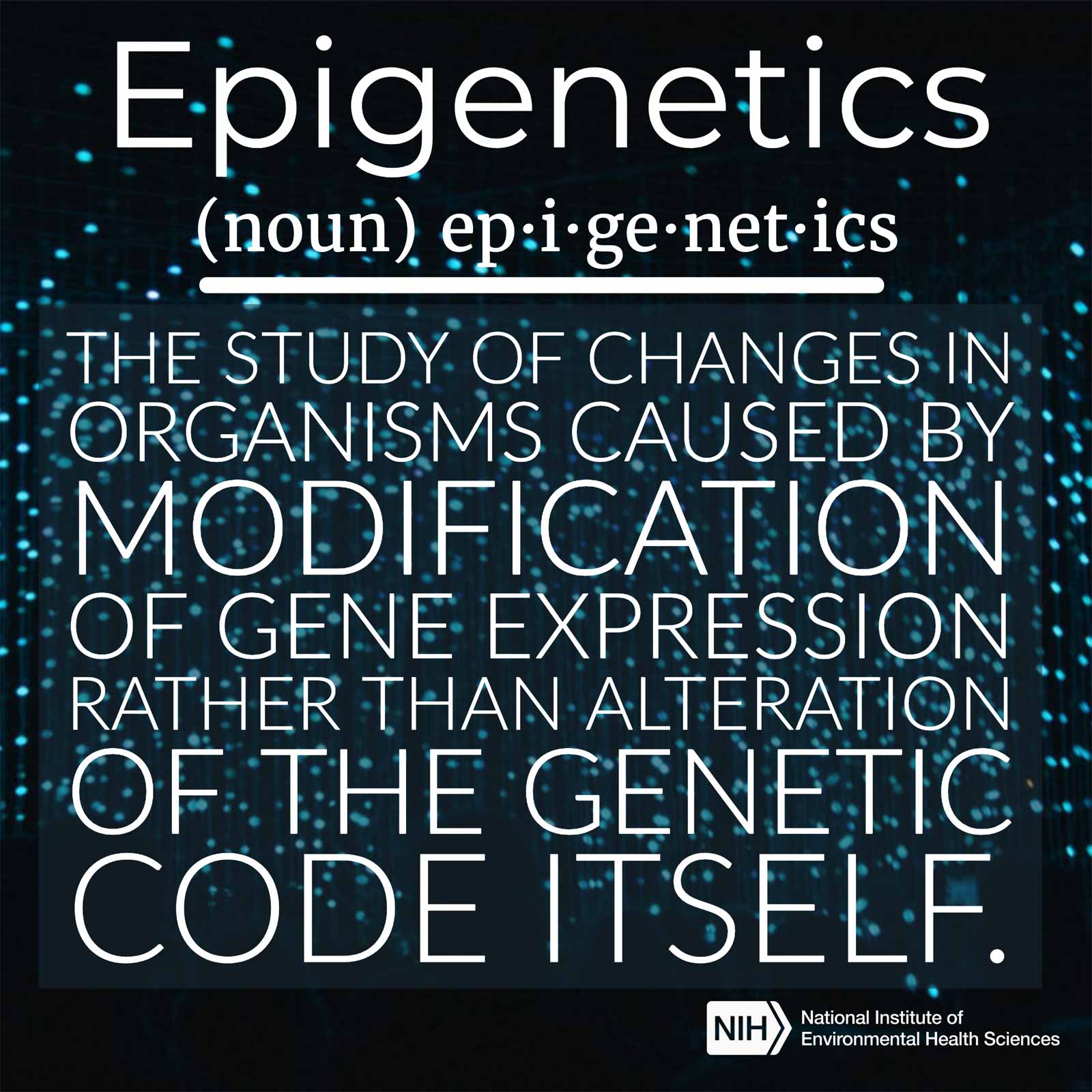
In future months, look for more interviews like this one. I want to increase opportunities for collaboration, which starts with conversation and the exchange of ideas. (Image courtesy of NIEHS)Her research into how chemical exposure during early life can interact with an individual’s epigenome and influence later-life health and disease is at the cutting edge of environmental health science. The epigenome is the collection of chemical modifications on DNA or the proteins associated with DNA that affect how genes are turned on and off.
Walker’s innovative research approaches and desire to promote collaboration dovetail with my core leadership values, outlined in my inaugural Director’s Corner column. A renowned scientist, Walker was elected to the National Academy of Medicine in 2016.
She is a member of the NIEHS Toxicant Exposures and Responses by Genomic and Epigenomic Regulators of Transcription (TaRGET) consortium. Walker also directs her center’s Training in Precision Environmental Health Sciences program.
I hope that our conversation sparks discussion among scientists, especially those who study the origins of disease or conduct biomedical research. Readers also will learn about Walker’s intellectual passion and what spurred her desire to become a researcher (see sidebar).
Individual susceptibility to disease
RW: You and I have talked in the past about moving beyond population statistics to develop a better understanding of how exposures can affect individual health and disease susceptibility. Statistics from large study groups are important for public health, but at the end of the day, I think that people reading this column want to know how the work we do relates to them and their unique biological makeup. This is where the idea of precision environmental health, which you have helped to pioneer, comes into play. Can you please explain that concept?
 “If we disrupt epigenetic programming during [key] windows of susceptibility, we carry a disturbed epigenome with us for the rest of our lives,” said Walker in 2019 at NIEHS. (Photo courtesy of Steve McCaw / NIEHS)
“If we disrupt epigenetic programming during [key] windows of susceptibility, we carry a disturbed epigenome with us for the rest of our lives,” said Walker in 2019 at NIEHS. (Photo courtesy of Steve McCaw / NIEHS)CW: In the past, testing drugs in patients to see which ones worked was based on a trial-and-error strategy. In recent years, as we have come to understand the basis for disease at the molecular level, more thought has been given to patient selection for trials and identifying who are the best candidates for a given drug.
Take cancer as an example. These days, in deciding which therapy is best, we do genomic profiling of tumors to determine whether an individual’s cancer has a specific defect or mutation that can be targeted with a particular drug. This is precision medicine — getting the right drug to the right patient to treat disease most effectively.
For precision environmental health, the precision part is really about understanding individual risk and preventing, rather than treating, disease.
The idea is to move from population- to individual-level risk estimates and enable effective prevention efforts. That requires analyzing genome and epigenome profiles; measuring exposures across the lifespan, collectively called the exposome; and incorporating powerful data science tools.
Epigenome, meet environment
RW: In 2019, you spoke at NIEHS as part of the institute’s Distinguished Lecture Series. You discussed an aspect of your research that I find especially fascinating — how early-life exposures can reprogram an individual’s epigenome and pave the way for ill health later on. What would you like people to know about this topic?
CW: The epigenome often is called the software of the genome, and that is absolutely true. If you change the software, you are going to change the way the genome functions. We are starting to see how the environment affects the epigenome and changes the way the body functions, which can increase disease risk. Exciting discoveries regarding epigenome-environment interactions have resulted from the TaRGET consortium, of which I am honored to be a member.
One such discovery is that epigenetic changes caused by early exposure to an environmental agent — such as bisphenol A (BPA), an endocrine-disrupting chemical — may remain silent until some later-life challenge occurs, such as another exposure. Here, the term silent refers to epigenetic changes that do not alter gene expression until triggered by later-life events.
This is important because it means we may not be able to make broad assumptions about risks from a given exposure based on gene expression alone. People who do not experience a later-life challenge may be at increased risk, but if that event never occurs, they will not develop disease.
An illustration comes from my team’s work examining how early-life exposure to BPA can result in epigenetic changes that remain silent until later exposure to a high-fat, Western diet. In an animal model, alterations in the epigenome caused by BPA exposure changed gene expression only after these animals were placed on that diet.
The BPA-exposed animals exhibited metabolic dysfunction and higher levels of serum cholesterol and lipids. Importantly, neither the BPA exposure nor the diet alone were sufficient to cause these changes.
Endocrine disruptors, epigenetic aging
RW: Very interesting how some exposures can be silent until there is a second trigger. What else would you like readers to know?
CW: What we are learning in the second phase of TaRGET is quite interesting. My team is trying to determine which parts of the epigenetic apparatus are particularly vulnerable to environmental exposures.
The epigenome has its own internal clock that reflects biological age, which can differ from chronological age. We are finding that early-life exposure to endocrine disruptors can affect normal epigenetic aging.
Such disruption may be an underlying mechanism of some disease. In one of our models, early-life exposure to the chemical tributyltin changed age-related gene expression. That change correlated with susceptibility to fatty liver disease and liver tumors.
Remarkably, when we translated what we learned to human data sets, we found that we could accurately classify disease severity in individuals with nonalcoholic fatty liver disease. We could even distinguish between people with liver cancer versus those with a normal liver.
 The epigenome changes throughout life, in response to environmental cues. Some exposures cause epigenetic changes that resemble those of an older individual, resulting in and epigenetic age older than chronological age. (Photo courtesy of Lightspring / Shutterstock.com)
The epigenome changes throughout life, in response to environmental cues. Some exposures cause epigenetic changes that resemble those of an older individual, resulting in and epigenetic age older than chronological age. (Photo courtesy of Lightspring / Shutterstock.com)Thus, it looks like the ability of the epigenome to change with age, what we call epigenetic plasticity, makes it vulnerable to early-life environmental exposures. That dynamic could be a major driver in developing disease.
This kind of research illustrates that environmental exposures can be used as tools to teach us things about biology we would not otherwise learn. I am grateful to NIEHS for its foresight in recognizing the importance of the epigenome in disease and supporting this consortium, which allows participants to draw on their collective expertise and advance environmental health science in such an innovative way.
Double-edged sword
RW: Any parting words for Environmental Factor readers?
CW: The epigenome’s capacity to change during development and the course of normal aging is a double-edged sword.
Genomic stability is a high priority for all organisms, and humans have many mechanisms in place to protect and maintain their genome and prevent mutations. The exact opposite is true for the epigenome, which changes dramatically over the lifespan. It is built to sense the environment, especially early in life, when it can adapt and provide a survival advantage under changing conditions.
However, that can be a vulnerability. Some environmental exposures alter the epigenome in ways that can actually promote disease. We do not yet know how many exposures work this way, so continuing to examine how our environment affects our epigenome should be at the top of our to-do list when it comes to understanding human health and working to prevent disease.
(Rick Woychik, Ph.D., is director of NIEHS and the National Toxicology Program.)
Check out these papers for more detail on the topics discussed above. — Ed.
Trevino LS, Dong J, Kaushal A, Katz TA, Jangid RK, Robertson MJ, Grimm SL, Ambati CSR, Putluri V, Cox AR, Kim KH, May TD, Gallo MR, Moore DD, Hartig SM, Foulds CE, Putluri N, Coarfa C, Walker CL. 2020. Epigenome environment interactions accelerate epigenomic aging and unlock metabolically restricted epigenetic reprogramming in adulthood. Nat Commun 11(1):2316.
Katz TA, Grimm SL, Kaushal A, Dong J, Trevino LS, Jangid RK, Gaitan AV, Bertocchio JP, Guan Y, Robertson MJ, Cabrera RM, Finegold MJ, Foulds CE, Coarfa C, Walker CL. 2020. Hepatic tumor formation in adult mice developmentally exposed to organotin. Environ Health Perspect 128(1):17010.
Trevino LS, Katz TA. 2018. Endocrine disruptors and developmental origins of nonalcoholic fatty liver disease. Endocrinology 159(1):20–31.
Foulds CE, Trevino LS, York B, Walker CL. 2017. Endocrine-disrupting chemicals and fatty liver disease. Nat Rev Endocrinol 13(8):445-457.
Wang Q, Trevino LS, Wong RL, Medvedovic M, Chen J, Ho SM, Shen J, Foulds CE, Coarfa C, O'Malley BW, Shilatifard A, Walker CL. 2016. Reprogramming of the epigenome by MLL1 links early-life environmental exposures to prostate cancer risk. Mol Endocrinol 30(8):856–871.