Federal efforts during the past year to reduce animal use in chemical safety testing were celebrated at a May 21 public forum(https://ntp.niehs.nih.gov/whatwestudy/niceatm/3rs-meetings/past-meetings/pubforum-2020/iccvamforum-2020.html) held by the Interagency Coordinating Committee on the Validation of Alternative Methods(https://ntp.niehs.nih.gov/pubhealth/evalatm/iccvam/) (ICCVAM).
The committee is comprised of agencies such as NIEHS and the U.S. Food and Drug Administration (FDA). Stakeholders include representatives from industry, academia, and animal welfare groups. More than 400 individuals attended the online meeting.
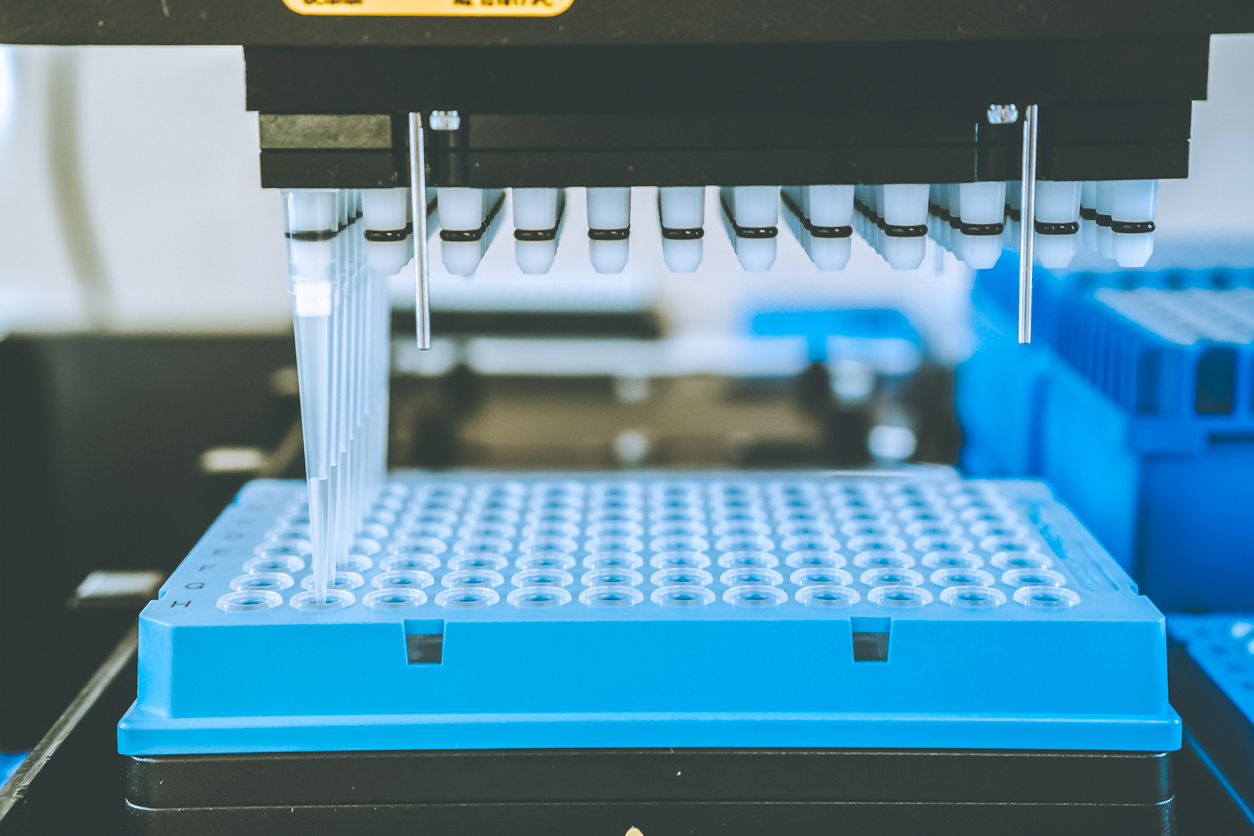 Automated machines such as this support high-throughput chemical screening, which is a nonanimal approach to toxicity testing.
Automated machines such as this support high-throughput chemical screening, which is a nonanimal approach to toxicity testing.Leadership key to new initiatives
“Things aren’t going to change unless they change at the top,” said ICCVAM Administrative Director Warren Casey, Ph.D. He praised NIEHS Scientist Emeritus Linda Birnbaum, Ph.D., for steering the group in a more productive direction in recent years (see sidebar). She is the former director of the institute and the National Toxicology Program (NTP).
 Casey’s team helps run the Tox21(https://ntp.niehs.nih.gov/whatwestudy/tox21/) program, which uses nonanimal methods to rapidly test chemicals for human health effects. (Photo courtesy of Steve McCaw)
Casey’s team helps run the Tox21(https://ntp.niehs.nih.gov/whatwestudy/tox21/) program, which uses nonanimal methods to rapidly test chemicals for human health effects. (Photo courtesy of Steve McCaw)Casey, who heads the NIEHS Biomolecular Screening Branch and until recently led the NTP Interagency Center for the Evaluation of Alternative Toxicological Methods(https://ntp.niehs.nih.gov/pubhealth/evalatm/) (NICEATM), commended the committee’s work groups(https://ntp.niehs.nih.gov/go/iccvam-wg) for their efforts to validate nonanimal testing approaches and engage with collaborators. “They have done an amazing job,” he said.
NICEATM, which organized the meeting, provides scientific and administrative support to ICCVAM. The annual forum is one of several ways the two groups share information with the scientific community and advance alternative testing.
“We’ve made a lot of progress in communication,” noted NICEATM Acting Director Nicole Kleinstreuer, Ph.D. “Getting stakeholder feedback is something that we really value.”
Saving animals, saving money
Last year, the U.S. Environmental Protection Agency (EPA) Office of Pesticide Programs saved approximately 1,500 animals and $1.8 million by waiving the use of animals in six toxicity studies. Monique Perron, Sc.D., from EPA, said that going forward, similar waivers will help the agency achieve its goal of reducing animal study requests and funding by 30% over the next several years (see sidebar of this November 2019 Environmental Factor article).
Suzanne Fitzpatrick, Ph.D., from FDA, highlighted efforts by leaders at her agency. “The Office of the Chief Scientist was able to bring in representatives from every single product center to work on alternatives,” she said. “We are accountable to the Office of the Commissioner every year to [show] what we’re doing.” In the last two years, FDA published six guidance documents that promote reduction of animal use in drug safety testing.
 Kleinstreuer helped represent ICCVAM at an April meeting convened by the Organisation for Economic Co-operation and Development. (Photo courtesy of Steve McCaw)
Kleinstreuer helped represent ICCVAM at an April meeting convened by the Organisation for Economic Co-operation and Development. (Photo courtesy of Steve McCaw)Mamta Behl, Ph.D., an NIEHS toxicologist, discussed nonanimal approaches to identify substances that can potentially harm brain function, focusing on flame retardants. One of her projects assesses chemical neurotoxicity through cell-based studies and zebrafish models, for example, rather than rodent tests.
Many groups represented
Representatives from other agencies also made presentations:
- Air Force 59th Medical Wing, U.S. Department of Defense.
- Animal Welfare Information Center, U.S. Department of Agriculture.
- EPA Office of Research and Development.
- EPA Office of Pollution Prevention and Toxics.
- National Center for Advancing Translational Sciences.
- National Institute of Standards and Technology.
“What is most striking to me today is to finally see a lot of payoff from years of collaboration,” said Amy Clippinger, Ph.D., who represented the PETA International Science Consortium, Ltd. “And now, most importantly, we’re seeing [new research methods] being used in regulatory decision-making,” she added.
(Catherine Sprankle is a communications specialist for ILS, the contractor supporting NICEATM.)