Of the approximately 28,000 plants that have medicinal properties, only a few have been assessed for safety. Scientists discussed an ambitious effort to fill that gap at the first public meeting of the Botanical Safety Consortium (BSC) on May 29.
More than 300 people from government, academia, industry, nonprofits, and consumer health groups met online to consider challenges and opportunities related to studying botanicals. The consortium is a partnership between NIEHS, the U.S. Food and Drug Administration (FDA), and the Health and Environmental Sciences Institute (HESI).
 Approximately 18% of people in the United States take nonvitamin, nonmineral dietary supplements.
Approximately 18% of people in the United States take nonvitamin, nonmineral dietary supplements.“We feel botanicals are of public health concern because of the widespread exposure of potentially vulnerable populations, such as those with preexisting conditions or older adults,” said Cynthia Rider, Ph.D. She is a toxicologist at NIEHS and a member of the consortium’s steering committee.
 Rider shared how the Toxicology in the 21st Century Program(https://ntp.niehs.nih.gov/whatwestudy/tox21/) could help advance the study of botanical safety. (Photo courtesy of Steve McCaw)
Rider shared how the Toxicology in the 21st Century Program(https://ntp.niehs.nih.gov/whatwestudy/tox21/) could help advance the study of botanical safety. (Photo courtesy of Steve McCaw)“Unlike many of the contaminants that we are typically concerned about, exposure to botanicals can occur at relatively high doses,” Rider added.
Overcoming research obstacles
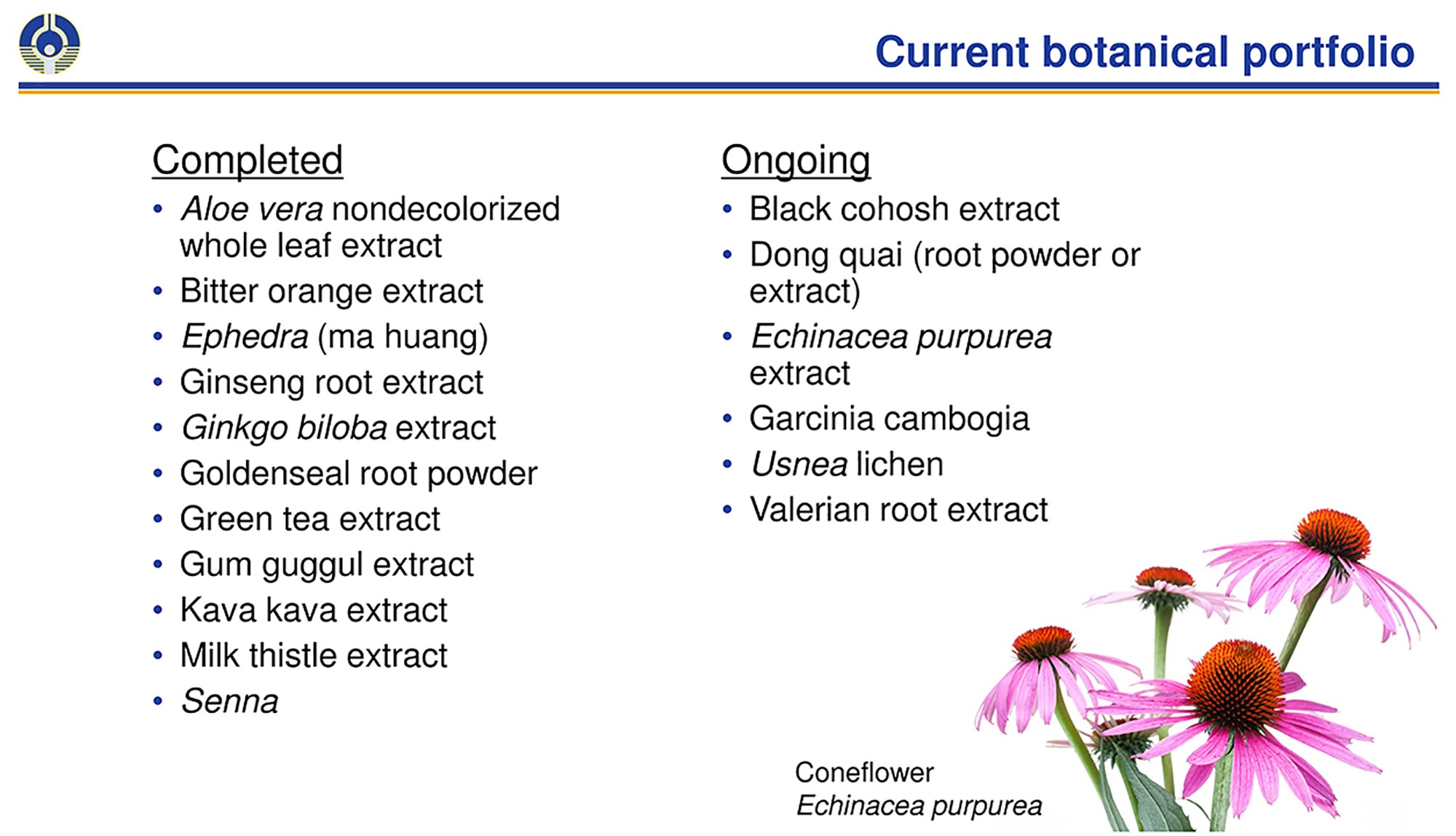
In the past 20 years, scientists in the NIEHS Division of the National Toxicology Program completed studies on aloe vera, ephedra, ginkgo biloba, green tea extract, milk thistle extract, and other substances. Researchers sought to overcome obstacles that arise from the sheer number and diversity of products available to consumers.
For example, the final commercial form of a given botanical can vary significantly, depending on how it is processed from raw plant material. That variation can make it difficult to determine whether safety data gathered from one version of a botanical applies to others on the market.
Rider and her colleagues addressed that problem by comparing the chemical and biological properties of different products containing black cohosh and Echinacea purpurea, which are two popular botanicals. The researchers’ technique helps to show whether a particular botanical mixture is sufficiently similar to a tested mixture for purposes of safety assessment.
“I think this is one of the approaches that will be very useful with the Botanical Safety Consortium moving forward,” she said.
Sharing expertise
Other scientists who participated in the meeting include the following:
- Dan Marsman, D.V.M., Ph.D., from Proctor and Gamble, gave an overview of botanicals and current challenges related to safety assessment.
- Cara Welch, Ph.D., from FDA, described efforts to modernize her agency’s Dietary Supplement Program.
- Joe Dever, Ph.D., from Amway Corporation, shared the purpose and progress of the consortium’s technical working groups (see sidebar).
- Stefan Gafner, Ph.D., from the American Botanical Council, presented technical approaches to studying plant chemistry and botanical ingredients.
- James Griffiths, Ph.D., from the Council for Responsible Nutrition, concluded with a description of the consortium’s goals and strategy for the future.
Open to the public
“[BSC seeks to] enhance the botanical safety toolkit and bring clarity to botanical dietary ingredient assessments for manufacturers and regulators,” said Connie Mitchell, HESI scientific program manager. Research publications and other materials will be made available to the public and stakeholders on the consortium’s website, she noted.
Mitchell presented both the history of BSC and a timeline for the year ahead. By the fall, group members hope to finalize a list of methods and ingredients to test as they create guidance for future studies on botanical safety.
Citation: Ryan KR, Huang MC, Ferguson SS, Waidyanatha S, Ramaiahgari S, Rice JR, Dunlap PE, Auerbach SS, Mutlu E, Cristy T, Peirfelice J, DeVito MJ, Smith-Roe SL, Rider CV. 2019. Evaluating sufficient similarity of botanical dietary supplements: combining chemical and in vitro biological data. Toxicol Sci 172(2):316–329.
(Marla Broadfoot, Ph.D., is a contract writer for the NIEHS Office of Communications and Public Liaison.)