 Lawler leads the NIEHS Genes, Environment, and Health Branch. (Photo courtesy of Steve McCaw)
Lawler leads the NIEHS Genes, Environment, and Health Branch. (Photo courtesy of Steve McCaw)April is Autism Awareness Month, so the Environmental Factor took a look back at related NIEHS-funded research. Several studies reported progress toward potential biomarkers for autism spectrum disorder (ASD).
“The results of NIEHS-supported autism biomarker research may inform clinical and public health practices,” said Cindy Lawler, Ph.D., who oversees the NIEHS autism grant program.
She explained that biomarkers of susceptibility can inform screening of infants and young children who may be at increased risk, because early intervention can be beneficial.
“Identifying biomarkers can also provide clues about the underlying biology of autism,” Lawler said. “There may be distinct subgroups, with different origins, that merit different strategies for prevention and intervention.”
New gene of interest
Researchers in the NIEHS Neurobiology Laboratory reported in November 2019 that when a gene called Nr3c2 was disrupted in mice, the resulting social behaviors and responses to new objects were similar to those often associated with ASD.
Postdoctoral fellow Katharine McCann, Ph.D., lead author of the study, said that Nr3c2 plays a key role in hormonal responses to a stressful experience. Therefore, disruption to its function or expression could have long-lasting consequences for stress-related behavior.
 McCann’s study focused on the Nr3c2 gene, which codes for a protein called the mineralocorticoid receptor. (Photo courtesy of Steve McCaw)
McCann’s study focused on the Nr3c2 gene, which codes for a protein called the mineralocorticoid receptor. (Photo courtesy of Steve McCaw)The gene is highly expressed in the brain region called the hippocampal area CA2, and appears to play significant roles in CA2 development.
Serena Dudek, Ph.D., deputy chief of the Neurobiology Laboratory and the study’s senior author explained that her group was working with the gene before its link with autism. Her team focuses on the CA2 region.
“CA2 had already been reported to be linked to social behavior, by us and others,” she said. “Nr3c2 is particularly interesting because it is not expressed to the same extent throughout the brain, like many other autism-linked genes are.”
Other recent studies reported that mutations in Nr3c2 defined a new ASD syndrome, and three brothers with autism shared the same type of Nr3c2 mutation.
Metabolism of metals, other elements
In September 2019, grantee Manish Arora, Ph.D., from Icahn School of Medicine at Mt. Sinai, co-authored a paper on metabolic regulation of nutrients and toxins and development of both ASD and attention deficit hyperactivity disorder (ADHD).
The researchers analyzed baby teeth of twins to study metabolism of essential and toxic elements. They found differences in exposures between children with typical neurodevelopment, or neurotypical, and children with ASD.
“Dysregulation of cyclical processes in elemental metabolism during prenatal and early postnatal development not only encompasses [metabolic] pathways shared by ADHD and ASD, but also comprise features specific to either condition,” the authors wrote.
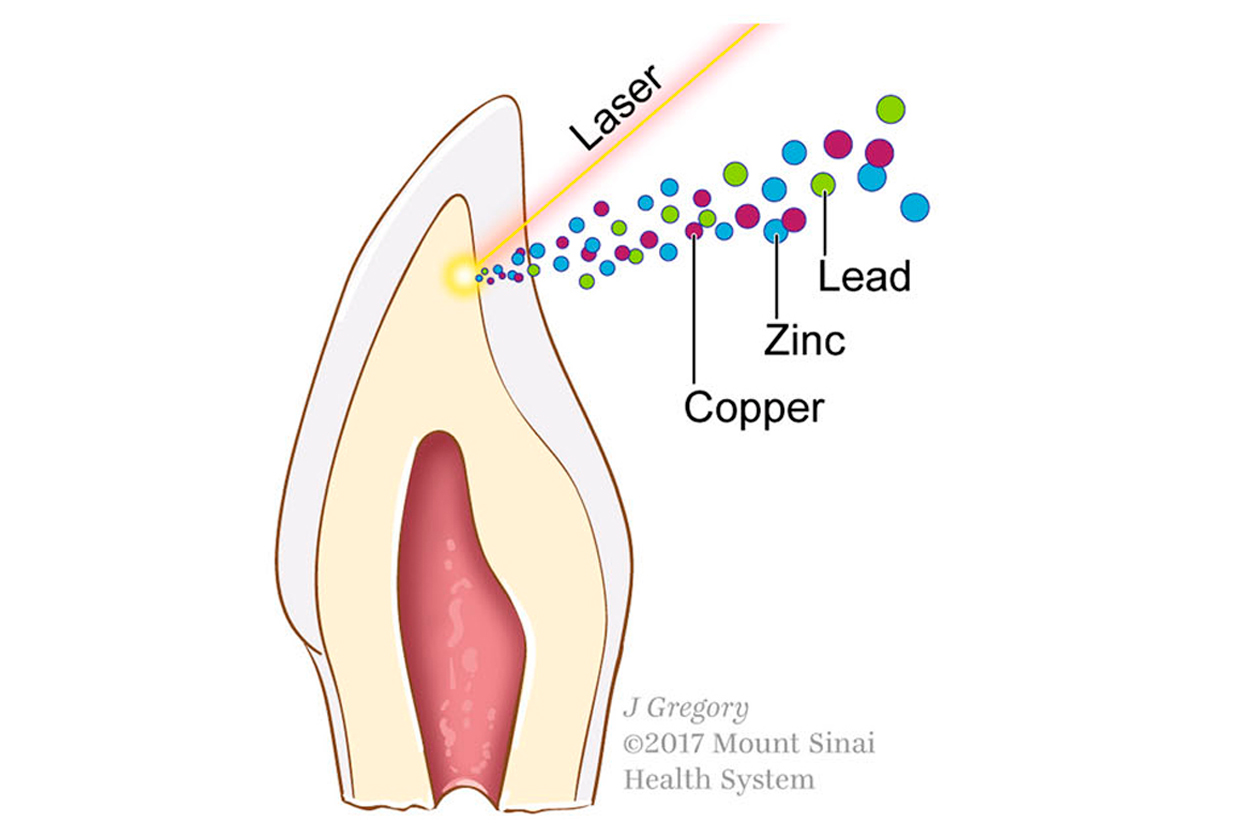 The team used lasers on baby teeth to generate profiles of metal uptake during fetal and postnatal development. (Image courtesy of Austin et al. through Creative Commons Attribution 4.0 International License)
The team used lasers on baby teeth to generate profiles of metal uptake during fetal and postnatal development. (Image courtesy of Austin et al. through Creative Commons Attribution 4.0 International License)Immune markers
A study funded in part by NIEHS looked at levels in neonatal blood of immune system components and observed increases in proinflammatory molecules known as IL-6 and IL-8.
“Elevated levels of some inflammatory markers in newborn bloodspots indicated a higher degree of immune activation at birth in children who were subsequently diagnosed with ASD,” the authors wrote. Expansion of their exploratory study might lead to identification of biomarkers for assessment of ASD risk in newborns.
Clues from the placenta
Researchers with the NIEHS-funded Markers of Autism Risk in Babies Learning Early Signs (MARBLES) study linked epigenetic changes in the placentas of newborns later diagnosed with ASD. Epigenetic changes, such as the addition of chemical tags called methyl groups, influence gene activity without altering the underlying DNA.
The scientists studied the placentas of more than 40 women and found 400 regions in the genome where methylation differed between those later diagnosed with ASD and neurotypical children. Further analyses revealed two epigenetic changes that may serve as biomarkers in very early life.
These differently methylated regions overlapped regions previously identified as areas of genetic risk for ASD. The researchers further noted that gene methylation was affected by use of prenatal vitamins.
Citations:
Austin C, Curtin P, Curtin A, Gennings C, Arora M, Tammimies K, Isaksson J, Willfors C, Bolte S. 2019. Dynamical properties of elemental metabolism distinguish attention deficit hyperactivity disorder from autism spectrum disorder. Transl Psychiatry 9(1):238.
Graciarena M. 2019. Cytokines and chemokines in novel roles: exploring their potential as predictors of autism spectrum disorder. Biol Psychiatry 86(4):e11–e12.
Heuer LS, Croen LA, Jones KL, Yoshida CK, Hansen RL, Yolken R, Zerbo O, DeLorenze G, Kharrazi M, Ashwood P, Van de Water J. 2019. An exploratory examination of neonatal cytokines and chemokines as predictors of autism risk: The Early Markers for Autism study. Biol Psychiatry 86(4):255–264.
McCann KE, Lustberg DJ, Shaughnessy EK, Carstens KE, Farris S, Alexander GM, Radzicki D, Zhao M, Dudek SM. 2019. Novel role for mineralocorticoid receptors in control of a neuronal phenotype. Mol Psychiatry; doi:10.1038/s41380-019-0598-7 [Online 19 November 2019].
Pagani JH, Zhao M, Cui Z, Avram SK, Caruana DA, Dudek SM, Young WS. 2015. Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Mol Psychiatry 20(4):490–499.
Zhu Y, Mordaunt CE, Yasui DH, Marathe R, Coulson RL, Dunaway KW, Jianu JM, Walker CK, Ozonoff S, Hertz-Picciotto I, Schmidt RJ. 2019. Placental DNA methylation levels at CYP2E1 and IRS2 are associated with child outcome in a prospective autism study. Human Mol Genet 28(16):2659–2674.









