Williams is head of the Structural Cell Biology Group at the NIEHS, and holds a secondary appointment in the Signal Transduction Laboratory. (Photo courtesy of Steve McCaw)
Williams is head of the Structural Cell Biology Group at the NIEHS, and holds a secondary appointment in the Signal Transduction Laboratory. (Photo courtesy of Steve McCaw)A protein called Ctp1 mediates the repair of DNA double-strand breaks by forming filaments that bridge two DNA strands, according to a study in yeast by NIEHS researchers and their colleagues at the University of North Carolina at Chapel Hill (UNC). The findings, published in the March 1 issue of the Journal of Biological Chemistry, could provide insight into the DNA repair defect that causes Seckel and Jawad syndromes in humans.
“Our DNA strands are continuously broken through intrinsic cellular DNA processes, and through environmental exposures to DNA-damaging radiation and chemotherapeutic drugs,” said senior study author Scott Williams, Ph.D., deputy chief of the NIEHS Genome Integrity and Structural Biology Laboratory.
“Ctp1 is a first responder to DNA damage, but the molecular nature of the Ctp1-DNA interaction was unknown,” he said. “This work provides the first molecular snapshot of the Ctp1 protein bound to DNA.”
Faulty repair leads to disease
To maintain the integrity of the genome, cells evolved complex mechanisms to detect DNA damage, signal its presence, and guide its repair. Inherited defects in these mechanisms can cause the death of an embryo or severe diseases. The effects of such diseases are wide-ranging and include delayed growth, mental retardation, skeletal abnormalities, and predisposition to cancer.
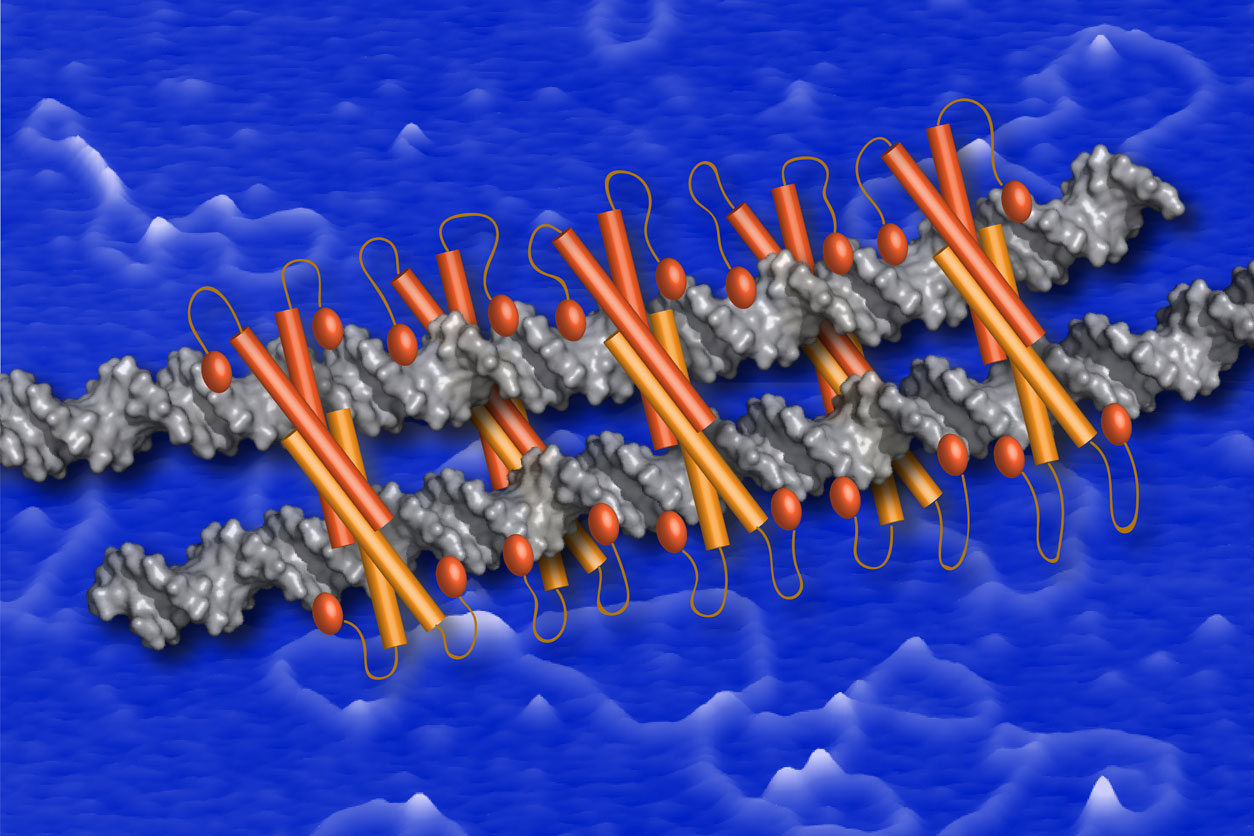 A model for the Ctp1-DNA bridging filament is depicted as orange Ctp1 protein tetramers bound to silver DNA strands, shown on a blue background of atomic force imaging. (Photo courtesy of Sara Andres and Scott Williams)
A model for the Ctp1-DNA bridging filament is depicted as orange Ctp1 protein tetramers bound to silver DNA strands, shown on a blue background of atomic force imaging. (Photo courtesy of Sara Andres and Scott Williams)In the yeast the researchers studied, Ctp1 is the equivalent of a protein in humans called CtIP. Mutations in CtIP cause Seckel and Jawad syndromes in humans, which are characterized by facial, skin, and finger abnormalities; developmental delays; and microcephaly — a birth defect in which a baby’s head is smaller than expected.
Scientists knew that Ctp1 plays an important role in repairing DNA double-strand breaks, but until now, the underlying mechanism was unclear, according to Petr Cejka Ph.D., from the Institute for Research in Biomedicine in Switzerland.
“This unanticipated role of Ctp1 might help explain how the processing of DNA ends is coordinated to facilitate DNA break repair,” wrote Cejka in the Editor’s Pick Highlight that accompanied the paper.
“We knew that Ctp1 could bind DNA and bridge two strands together, but we didn’t know how,” Williams explained. “Answering this question could ultimately shed light on the underlying cause of Jawad and Seckel syndromes in humans.”
Seeing Ctp1 in action
To address this gap in knowledge, Williams and his team collaborated with Dorothy Erie, Ph.D., and her lab at UNC to use atomic force microscopy imaging to observe how Ctp1 interacted with DNA. While it was bound to double-stranded DNA, Ctp1 clustered into tetramers, or protein complexes consisting of four subunits. Each tetramer had an average length of approximately 15 base pairs. Base pairs are the building blocks of the DNA double helix.
These Ctp1 tetramers formed filaments that each spanned approximately 180 base pairs. These filaments bridged two double-stranded DNA molecules. “Protein–DNA filaments are an architectural feature frequently found to regulate genomic structure and repair,” Williams noted.
 As postdoctoral fellow at NIEHS from 2012 to 2017, Andres (see sidebar) learned how to use atomic force microscopy to visualize Ctp1 filaments bridging DNA for the first time. (Photo courtesy of Steve McCaw)
As postdoctoral fellow at NIEHS from 2012 to 2017, Andres (see sidebar) learned how to use atomic force microscopy to visualize Ctp1 filaments bridging DNA for the first time. (Photo courtesy of Steve McCaw)To mediate zipping the DNA, multiple Ctp1 tetramers lined up next to one another. Mutations that prevented Ctp1 from forming multiunit complexes severely impaired DNA bridging. Moreover, mutations that changed the DNA bridging made cells especially sensitive to radiation and chemicals that produce double-strand breaks.
“We believe that the DNA binding and bridging activity of Ctp1 has likely been conserved in evolution and plays a similar role in humans,” Williams said.
Next steps
According to Williams, the next step is to obtain a detailed molecular understanding of the Ctp1-DNA interaction, using the new NIEHS cryo-electron microscopy facility.
“These initial molecular snapshots of Ctp1-DNA complexes provide the first steps toward understanding how we might directly control DNA repair processes mediated by Ctp1,” Williams said.
“If we can influence CtIP functions in human cells, for instance, through small molecules that impede its functions, such compounds could sensitize tumor cells to chemotherapies, or control gene editing outcomes,” he suggested.
Citations:
Andres SN, Li ZM, Erie DA, Williams RS. 2019. Ctp1 protein-DNA filaments promote DNA bridging and DNA double-strand break repair. J Biol Chem 294(9):3312–3320.
Cejka P. 2019. Seeing is believing: DNA zipping promotes DNA repair. J Biol Chem 294(9):3321–3322.
Qvist P, Huertas P, Jimeno S, Nyegaard M, Hassan MJ, Jackson SP, Borglum AD. 2011. CtIP mutations cause Seckel and Jawad syndromes. PLoS Genet 7(10):e1002310.
(Janelle Weaver, Ph.D., is a contract writer for the NIEHS Office of Communications and Public Liaison.)