If you have read the book or watched the movie “Wonder,” which tells the story of a 10-year-old boy named Auggie with a facial abnormality, you may be familiar with a rare birth defect called Treacher Collins Syndrome (TCS). In this genetic disorder, the bones of the head and face, or craniofacial region, grow abnormally during early embryonic development.
Paul Trainor, Ph.D., a researcher from the Stowers Institute for Medical Research in Kansas City, Missouri, studies the cellular and genetic origin of human craniofacial defects such as TCS. He spoke Dec. 8 as part of the NIEHS Distinguished Lecture series. NIEHS Lasker Clinical Research Scholar Natalie Shaw, M.D., hosted the virtual event.
 Trainor's team demonstrated that, in contrast to classical models, cranial NCCs in mouse embryos are plastic; their development can be influenced by tissues they interact with during migration. (Photo courtesy of Paul Trainor / Stowers Institute for Medical Research)
Trainor's team demonstrated that, in contrast to classical models, cranial NCCs in mouse embryos are plastic; their development can be influenced by tissues they interact with during migration. (Photo courtesy of Paul Trainor / Stowers Institute for Medical Research)Focus on prevention, not repair
Abnormal development of the head and face accounts for one-third of all birth defects, according to Trainor. Among the more than 700 distinct craniofacial syndromes, TCS is quite rare. It is characterized by a small jaw, cleft lip or palate, and middle and external ear defects.
Physicians focus on repair, either by surgery or tissue engineering. The U.S. Centers for Disease Control and Prevention estimates that $700 million is spent each year on patients to repair cleft lips and cleft palates.
Trainor’s goal is to shift the focus toward prevention. “There is a very fine line between normal and abnormal craniofacial development,” he said. “If you can understand the genetic, cellular, and developmental basis of congenital defects, you can come up with creative and potentially preventative solutions,” Trainor added.
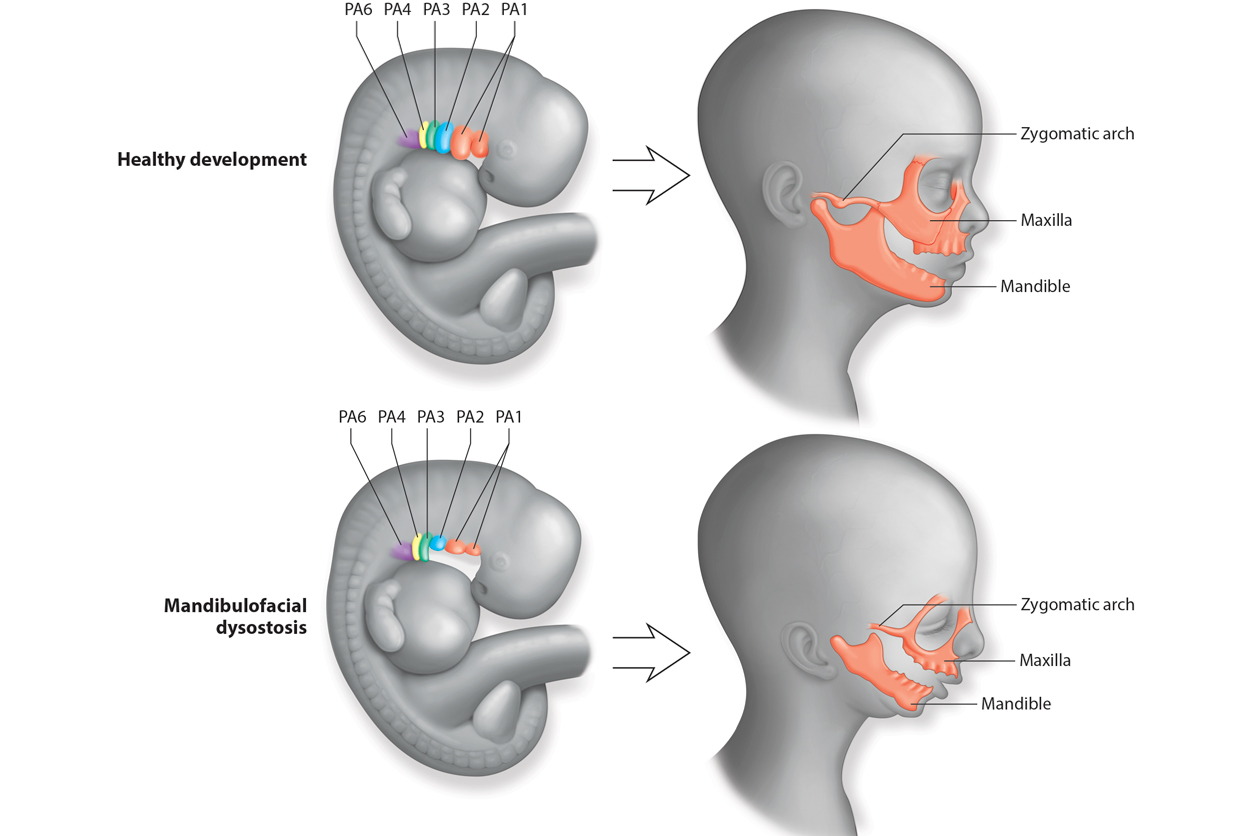 Healthy development, top, leads to familiar facial structures. In an embryo with TCS, or mandibulofacial dysostosis, facial structures do not develop normally. (Image used by permission of Paul Trainor, from Terrazas et al., 2017, Wiley Interdiscip Rev Dev Biol 6(3):10.1002/wdev.263)
Healthy development, top, leads to familiar facial structures. In an embryo with TCS, or mandibulofacial dysostosis, facial structures do not develop normally. (Image used by permission of Paul Trainor, from Terrazas et al., 2017, Wiley Interdiscip Rev Dev Biol 6(3):10.1002/wdev.263) Neural crest cells crucial
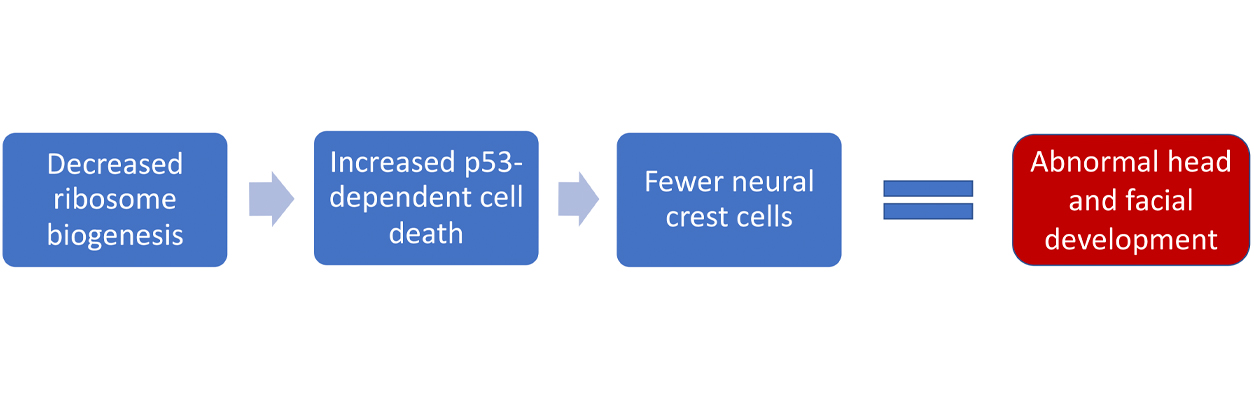
Using mouse and zebrafish models, Trainor’s group discovered that a shortage of specialized cells called neural crest cells during early pregnancy causes TCS and other craniofacial defects. Neural crest cells give rise to most of the facial bones, cartilage, and connective tissues of the head and face.
The group found that when a gene named TCOF1 is mutated, an embryo’s neural stem cells — which make neural crest cells — die. The team also found that blocking another gene that codes for a protein called p53, which promotes cell death, can restore the neural crest cell population.
TCOF1 encodes a protein called Treacle. Reduced amounts of Treacle lead to both a lower production, or biogenesis, of protein-producing organelles called ribosomes and to less repair of DNA damage.
Source of symptom variability
TCOF1 mutations can explain how TCS develops but not the variation observed among patients with TCS. Severity depends on other factors, such as DNA repair. Trainor’s team found that DNA repair is delayed in TCOF1-deficient mice.
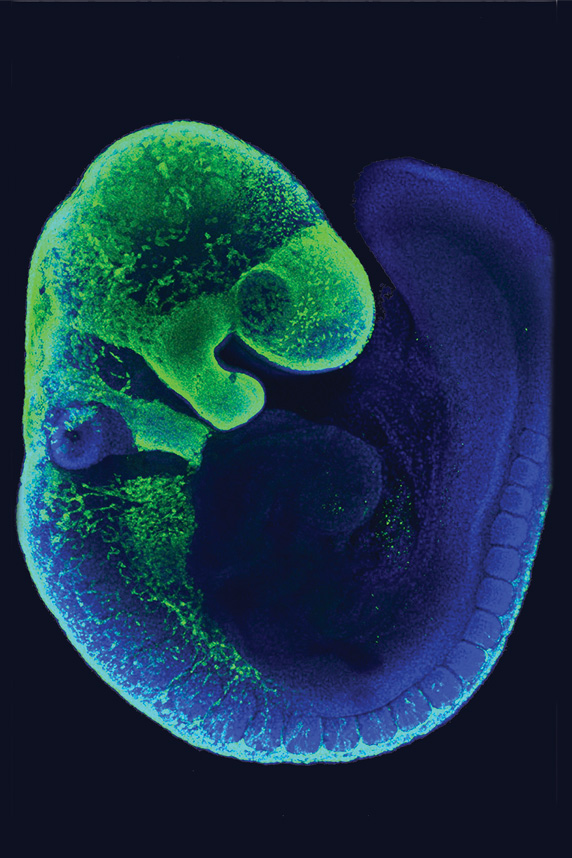 In this mouse embryo, migrating NCCs are shown in green. (Image courtesy of Amanda Barlow and Paul Trainor / Stowers Institute for Medical Research)
In this mouse embryo, migrating NCCs are shown in green. (Image courtesy of Amanda Barlow and Paul Trainor / Stowers Institute for Medical Research)Oxidative stress, which involves molecules called reactive oxygen species (ROS), appears to play a role in this delay. As Trainor’s team suspected, treatment with a diet high in antioxidants reduced oxidative stress in the mouse embryos. Individuals with mutations in TCOF1 are more likely to display severe symptoms if they are exposed to high levels of ROS in utero.
Genetics, not parents’ bad luck
Over the years, Trainor has met many families who are affected by birth defects such as TCS. Being able to explain to a family how this developmental disorder happens means a lot to them, as well as to him. “Parents feel an incredible amount of guilt when they have a child with a developmental disorder,” Trainor said.
“The first thing they do is blame themselves. That is a very natural thing to do in the absence of scientific information to explain that it actually had nothing to do with them,” he added. “Rather, it is the vagary of genetics.”
Through his research, he hopes to develop creative ways to prevent congenital birth defects in babies.
(Arif Rahman, Ph.D., is a visiting fellow in the NIEHS Toxicoinformatics Group.)