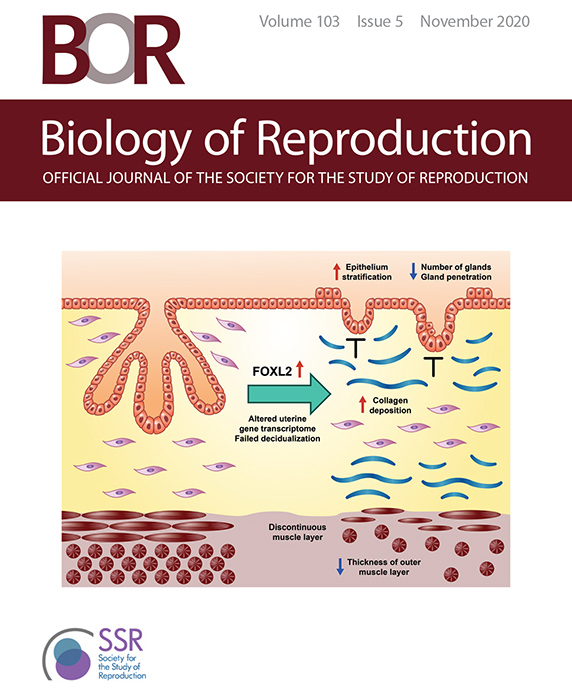
 Biology of Reproduction's November 2020 cover featured a graphical abstract of DeMayo’s paper. (Image used by permission of the Society for the Study of Reproduction)
Biology of Reproduction's November 2020 cover featured a graphical abstract of DeMayo’s paper. (Image used by permission of the Society for the Study of Reproduction)Researchers from the NIEHS Reproductive and Developmental Biology Laboratory have further underscored the importance of FOXL2, a transcription factor, in women’s ovarian physiology and fertility. The results also clarify the role excess FOXL2 production can play in endometriosis. Their findings were published Oct. 29 as companion articles in the journal Biology of Reproduction and highlighted on the issue’s cover.
Transcription factors are proteins that control how genetic information from DNA is given to messenger RNA, which then delivers instructions to the cell for making a specific protein, such as FOXL2.
Lab chief Francesco DeMayo, Ph.D., who also leads the Pregnancy and Female Reproduction Group, and Humphrey Yao, Ph.D., head of the Reproductive Developmental Biology Group, both study how FOXL2 regulates gene expression in the female reproductive system, including pituitary glands, ovaries, and uterus.
In humans, FOXL2 genes that mutate or lose their function are associated with premature infertility and ovarian cancer. Expression of FOXL2 is increased in the uterine tissue of women with endometriosis, which can be an extremely painful disorder. As a hormone-dependent disease, endometriosis — in which uterine endometrial cells grow outside the uterus — can be influenced by endocrine disruptors in the environment.
Altering endometrial gland development
 Li’s study showed that increasing FOXL2 expressions in the female reproductive tract can disrupt ovarian and uterine functions. (Photo courtesy of Steve McCaw / NIEHS)
Li’s study showed that increasing FOXL2 expressions in the female reproductive tract can disrupt ovarian and uterine functions. (Photo courtesy of Steve McCaw / NIEHS)Lead author Rong Li, Ph.D., a contractor in DeMayo’s Pregnancy and Female Reproduction Group, worked with him to generate mice that expressed FOXL2 in cells from the female reproductive tract that respond to the hormone progesterone.
Too much FOXL2 altered how uterine epithelial cells differentiated and functioned. Epithelial cells create protective barriers around organs, blood vessels, the skin, and other tissues. When the uterine epithelial cells do not form correctly, endometrial cells can grow outside of the uterus.
In addition, endometrial stromal cells, which regulate the vascular structure of the uterus, showed increased fibrosis, which interfered with development of the endometrial glands.
“Using genetically engineered mice, this study identified pathways regulated by FOXL2 in the uterus that are similar to pathways elevated in uterine samples of women with endometriosis,” DeMayo said. “This research may allow for development of better diagnostic and therapeutic tools for the treatment of this widespread disease.”
Preventing ovulation
Female reproductive functions rely on the ovary’s capacity to produce eggs and secrete sex steroids. Barbara Nicol, Ph.D., from Yao’s group, led a study to understand the effects of irregular FOXL2 activity on female reproductive physiology and disorders. They created a mouse model that produced excess FOXL2 in reproductive organs.
 Nicol was the lead author on Yao’s study and a co-author on DeMayo’s paper. (Photo courtesy of Steve McCaw / NIEHS)
Nicol was the lead author on Yao’s study and a co-author on DeMayo’s paper. (Photo courtesy of Steve McCaw / NIEHS)By studying effects on ovarian development and function from the fetal stage through adulthood, the scientists discovered that excessive FOXL2 production made it harder for certain cells to differentiate. As a result, the follicles of female mice did not form correctly. The follicles, which secrete hormones and influence the stages of the estrus cycle, also showed defects in steroid production.
These females were infertile because they could not ovulate. This study reveals that fine-tuned production of FOXL2 is absolutely essential for proper ovarian physiology and fertility.
“We have identified a potential cause of ovarian disorders in women, which might provide a possible treatment target for such disorders,” Yao said.
Citations:
Li R, Wu SP, Zhou L, Nicol B, Lydon JP, Yao HHC, DeMayo FJ. 2020. Increased FOXL2 expression alters uterine structures and functions. Biol Reprod 103(5):951–965.
Nicol B, Rodriguez K, Yao HHC. 2020. Aberrant and constitutive expression of FOXL2 impairs ovarian development and functions in mice. Biol Reprod 103(5):966–977.
(Kelley Christensen is a contract writer and editor for the NIEHS Office of Communications and Public Liaison.)